Serum Institute of India (SII) may soon start Phase-III trials of another Covid-19 vaccine being developed by US-based biotechnology company Novavax.
SII is seeking regulatory nod from the Indian government to start the trials for this vaccine. Company sources said it can start the trials by January 2021 once the regulatory approval is received.
This will be Serum’s second Covid-19 vaccine to go for human trials in the country. SII’s first agreement is with AstraZeneca and Oxford University for the Covid-19 vaccine, Covishield. SII is awaiting clearance from the Drugs Controller General of India (DCGI) for emergency use authorisation of Covishield. Phase-III human trials of the Covishield vaccine in India is nearing completion.
Novavax’s development is two months behind that of AstraZenca.
SII has an agreement with Novavax to manufacture the NVX-CoV2373 Covid-19 vaccine. It targets manufacturing 1 billion doses in 2021. Novavax signed an agreement with SII in September, with plans to bring all the planned capacity online by mid-2021.
In May 2020, SII had sold its manufacturing facilities in Europe to Novavax for around $167 million in an all-cash deal. Novavax had acquired Praha Vaccines, based in Czech Republic, and part of the Cyrus Poonawalla Group with annual capacity of making 1 billion doses of Covid-19 vaccine antigen. The Praha Vaccines acquisition was supported by Novavax’ funding arrangement with the Coalition for Epidemic Preparedness Innovations (CEPI).
At full capacity, Novavax will be able to increase its global production capacity across three continents to over 2 billion doses annually. It has secured $2 billion in funding for its global coronavirus vaccine programme, including up to $388 million from CEPI.
SII’s expertise to scale up and manufacture NVX-CoV2373 will help ensure the supply of this most-needed vaccine, Adar Poonawalla, CEO of SII, had said while signing the agreement.
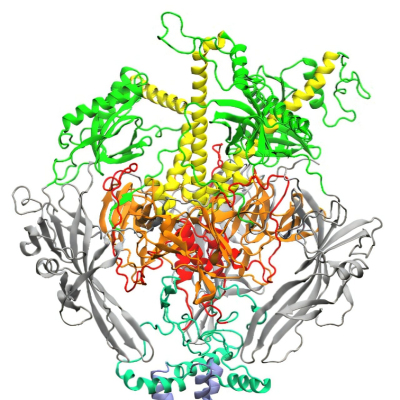
NVX CoV2373 is a vaccine candidate engineered from the genetic sequence of SARS CoV 2, the virus that causes Covid-19. It was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike (S) protein and contains Novavax’ patented saponin-based Matrix-M adjuvant. The antigen component of NVX-CoV2373 is being manufactured at SII.
Novavax had last month announced that the Covid-19 vaccine Phase-III trial process was starting in the US, Mexico and UK. Interim data from some of these trials were expected by the first quarter of 2021. This data was expected to serve as the basis for licensure application in the UK, European Union and other countries. Novavax was awarded $1.6 billion in funding by the US government to expedite the delivery of the vaccine doses.
Read the original article on The Financial Express.