Chronic infected wounds are often highly problematic for diabetic patients. However, a team of Chinese researchers have now developed a targeted approach to wound healing that makes use of nanomedicine, and their research has been published in the journal Angewandte Chemie. The researchers were able to deactivate wound-infecting bacteria using a solution of nanocapsules that alter the wound environment and unleash reactive oxygen species.
Chronic wounds in diabetic patients are an ideal place for bacteria to grow. The glucose-rich environment allows bacteria to form biofilms, making it very difficult for antibiotics to get to where they are needed. In addition, patients with diabetes often have weakened immune systems. In these cases, chemodynamic therapy offers a promising approach. Reactive oxygen species generated in situ weaken and damage the bacterial cells, causing them to die.
Chronic wounds in diabetic patients are an ideal place for bacteria to grow. The glucose-rich environment allows bacteria to form biofilms, making it very difficult for antibiotics to get to where they are needed. In addition, patients with diabetes often have weakened immune systems. In these cases, chemodynamic therapy offers a promising approach. Reactive oxygen species generated in situ weaken and damage the bacterial cells, causing them to die.
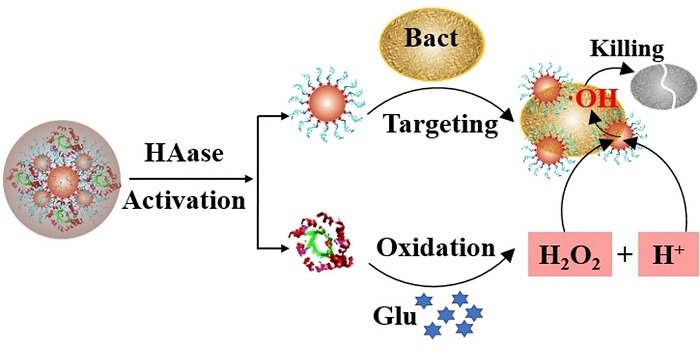
The main issue is that the catalyst can only break the hydrogen peroxide down in an acidic environment (i.e., at a low pH). However, most diabetic wounds are alkaline. To enable the nanozyme system to still be effective under these conditions, Ronghua Yang of Changsha University of Science and Technology in Changsha (China), and colleagues, dipped into their biochemistry bag of tricks and made use of the glucose-rich environment of diabetic wounds.
The microbial enzyme glucose oxidase, which is already known in medical diagnostics and the food industry, uses oxygen to convert glucose to gluconic acid, forming hydrogen peroxide and an acidic solution. Yang and the team attached glucose oxidase to the nanozymes, then embedded the whole system in a protective shell of hyaluronic acid.
The shell not only allowed the nanozyme particles to grow approximately five-fold to 0.1 micrometers (about a tenth of the size of a bacterium), it also kept them stable and unaltered in solution for more than 30 days. The hyaluronic acid shell served yet another purpose: bacteria produce enzymes that decompose hyaluronic acid, meaning the bacteria essentially unleash the tools of their own demise.
The nanocapsule solution was tested on bacterial cultures of Staphylococcus aureus, and killed the bacteria within a few hours. The team then treated chronic infected wounds in diabetic mice, and the results were decisive: under identical conditions, only the wounds treated with the nanocapsule solution healed completely and quickly.
The authors emphasized that the method did not require the synthesis of new materials; rather, they “solved physiological limitations on nanozymes by regulating the local microenvironment”. They also suggested that modifications of this type would be suitable for other nanozyme systems.
Read the original article on Wiley Online Library.