One degree (Celsius) can make a difference: a team of investigators led by Osaka University has demonstrated regulation of a critical protein complex that occurs only at physiological temperature.
To study the functions of various proteins, investigators generally examine purified proteins in vitro. Such experiments are generally limited to temperatures below physiological temperature to prevent thermal damage to the proteins. The team has overcome this problem by applying optically controlled heating with a pulsed infrared laser. “With this method, proteins can be heated up to 40°C without any damage to the proteins,” says lead author Hiroaki Kubota, “which enables us to examine how proteins act at their physiological temperature.”
The investigators applied this heating method to study the actomyosin complex. Actomyosin force generation, which results from the interaction between myosin molecular motors and actin filaments, is a crucial process governing cell motility and morphology. The protein drebrin E has been shown to inhibit these interactions, but its mechanism of regulation is unclear. To clarify the role of drebrin E, the team applied microscopic heating to actomyosin in the presence of drebrin and observed the motility produced by actomyosin force generation over a range from room temperature to physiological temperature.
The researchers found that the inhibition of actomyosin motility by drebrin E is eliminated immediately and reversibly during heating, depending on the drebrin E concentration. As the temperature increases, the inhibition of actomyosin motility by drebrin E decreases. The team also found that the reduced inhibition is not solely caused by dissociation of drebrin E from actin. Instead, bound drebrin E cooperatively regulates actomyosin activity likely by altering the actin structure.
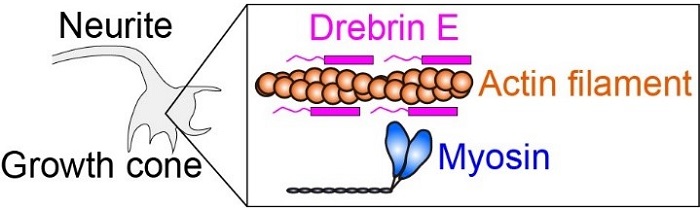
Fig.1 Intracellular force is produced by myosin interacting with actin filament (protein filament). Drebrin E can inhibit the actin-myosin interaction, hence regulate the intracellular force. It has been known that the concentration of drebrin E decreases during animal development. However, a correlation between the drebrin concentration and the actin-myosin activity has remained unclear, especially at the physiological temperature.
Interestingly, below physiological temperature, the actomyosin motility shows a gradual decay with increasing drebrin E concentration. Yet, at 36.5°C–37.5°C, the behavior changes, and drebrin E exhibits a switch-type behavior of inhibition. The motility is unaffected by the drebrin E concentration until a threshold is reached, which immediately results in inhibition. Their findings suggest that the threshold concentration is similar to previously reported physiological drebrin E concentrations.
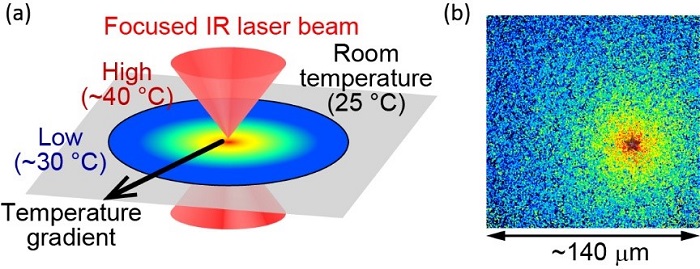
Fig.2 (a) Illustration of the optically-controlled microscopic heating method. (b) Localized temperature is visualized using fluorescent molecules. The heat source is located at the "star," where the temperature is measured as approximately 40 °C. The end of the field of view is approximately 30 °C. The size of the field of view is about 140 μm.
This temperature-dependent regulation of drebrin E is remarkable and has many implications for future studies. “These results suggest the importance for pregnant mammals to maintain the appropriate body temperature to ensure precise development of an embryo,” says Madoka Suzuki, senior author. Furthermore, gaining a precise understanding of the mechanisms of cellular temperature-sensing machinery, we may be able to innovate the development of artificial nanoscale temperature sensors.
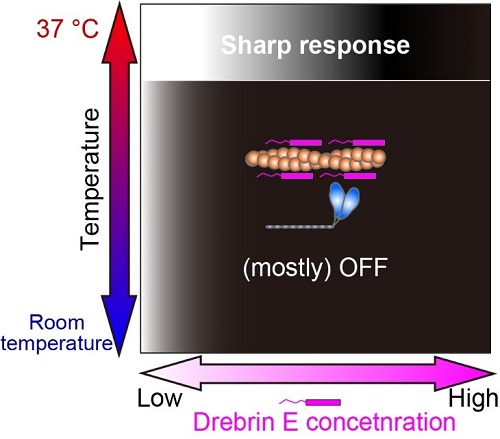
Fig.3 Our study revealed that the regulation of actin-myosin interaction by drebrin E is efficient only at the physiological temperature (37°C). The white-black gradation represents the force production On (white) and Off (black).
The article, “Microscopic temperature control reveals cooperative regulation of actin–myosin interaction by drebrin E” was published in Nano Letters.
Read the original article on Osaka University.