The key to chemical reactions is in the name — there needs to be something that causes the chemicals to react to one another. Called a catalyst, this component induces or speeds up reactions in a controlled manner to produce a desired outcome.
The catalysts used in several industries are often composed of noble metals, which are not efficient enough to compensate for their high cost. To address this issue for the chemical reaction of adding hydrogen, called hydrogenation, to quinoline, a molecule important in pharmaceutical production, researchers based in China have developed a highly effective catalyst comprising synergistic nanoparticles and single atoms of iridium.
They published their approach on March 22 in Nano Research.
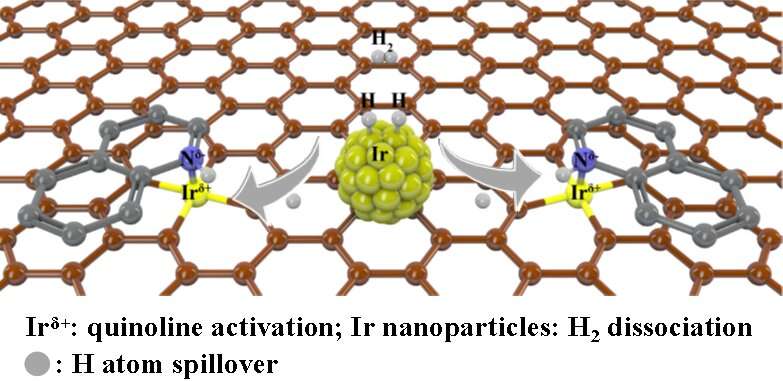
Synergistic iridium single atoms and nanoparticles catalyst exhibits unprecedentedly activity for quinoline hydrogenation.
“Selective hydrogenation of quinoline and its derivatives to the corresponding products have wide applications in fine chemical and pharmaceutical industry,” said co-corresponding author Changyan Cao, researcher in the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) and University of Chinese Academy of Sciences (UCAS). “Quinolines are an important class of compound for accessing tetrahydroquinoline products that are widely found in drug molecules, but noble metal catalysts are usually needed to produce this reaction. As such, it is highly important to improve the activity and the utilization efficiency of precious metals due to their high price.”
The researchers focused on single-atom catalysts, which Cao said have become a hot topic in the catalysis field due to how they can merge the advantages of both homogeneous and heterogeneous catalysts. The homogeneous catalysts encourage a uniform reaction, but heterogeneous catalysts can induce a higher yield reaction. The problem, according to Cao, is that single-atom catalysts lack a metal-metal bond. Without this bond to merge with, hydrogen is forced through a different pathway that results in less overall hydrogenation.
“Since hydrogen dissociates in matching pairs more easily on noble metal nanoparticles — to hydrogen atoms, for example — and it is well known that hydrogen atoms spillover, we hypothesized that hydrogen atoms formed on metal nanoparticles could also migrate to metal single sites for hydrogenation,” Cao said, explaining that the initial hydrogenation activity between the proposed catalyst and the substrate would essentially produce a secondary phase of hydrogen atoms capable of continuing the catalysis process. “By such design, the above-mentioned problems might be solved.”
To design such a catalyst, the researchers dispersed single atoms of iridium — the noble metal of highest reported intrinsic activity for hydrogenation of quinoline — and nanoparticles in a carbon support. When quinoline was applied, the reaction proved more efficient than when only iridium atoms or only nanoparticles were used.
“Constructing a synergistic catalyst changes the reaction path to take advantage of single atom sites in activation of substrate and nanoparticles in dissociation of hydrogen,” said co-corresponding author Weiguo Song, professor at ICCAS and UCAS. “All these features together contribute to the much-enhanced hydrogenation performance compared to the counterpart single-atom catalyst and nanoparticle catalyst alone.”
While the developed synergistic catalyst does not erase the need for noble metals, it does reduce the amount needed for a better reaction.
“We proposed and confirmed an efficient strategy to boost the catalytic activity for hydrogenation of quinoline by constructing a synergistic catalyst of iridium single atoms and nanoparticles, solving the activity limitations of single-atom catalysts,” Song said. “Next, we will expand our research to other metal catalysts and hydrogenation reactions to demonstrate the universality of synergist catalysis.”
Read the original article on EurekAlert.