Chemical separation processes are essential in the manufacturing of many products from gasoline to whiskey. Such processes are energetically costly, accounting for approximately 10–15% of global energy consumption. In particular, the use of so-called “thermal separation processes,” such as distillation for separating petroleum-based hydrocarbons, is deeply ingrained in the chemical industry and has a very large associated energy footprint. Membrane-based separation processes have the potential to reduce such energy consumption significantly.
Membrane filtration processes that separate contaminants from the air we breathe and the water we drink have become commonplace. However, membrane technologies for separating hydrocarbon and other organic materials are far less developed.
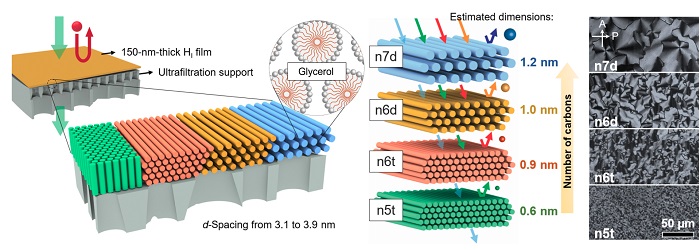
The researchers demonstrate how the methods used to create their membranes allow for fine-tuning the spacing of the nanostructures within the resulting filter.
Researchers from Penn’s School of Engineering and Applied Science are developing new membranes for energy-efficient organic separations by rethinking their physical structure on the nanoscale.
Nanofiltration using self-assembling membranes has been a major research area for Chinedum Osuji, Eduardo D. Glandt Presidential Professor in the Department of Chemical and Biomolecular Engineering, and his lab. The performance of these membranes was highlighted in a previous study describing how the structure of the membrane itself helped to minimize the limiting tradeoff between selectivity and permeability that is encountered in traditional nanofiltration membranes. This technology was also included in last year’s Y-Prize competition, and the winners have advanced a case for its use to produce non-alcoholic beer and wine in a startup called LiberTech.
Now, Osuji’s latest study adapts the membrane for filtration in organic solutions such as ethanol and isopropyl alcohol, and its self-assembling molecules make it more efficient than traditional organic-solvent nanofiltration (OSN).
The study, published in Science Advances, describes how the uniform pores of this membrane can be fine-tuned by changing the size or concentration of the self-assembling molecules that ultimately form the material. This tunability now opens doors for the use of this membrane technology in solving more diverse real-world organic filtration problems.
Read the original article on University of Pennsylvania.







