A team of researchers at Northwestern University has devised a new platform for gene editing that could inform the future application of a near-limitless library of CRISPR-based therapeutics.
Using chemical design and synthesis, the team brought together the Nobel-prize winning technology with therapeutic technology born in their own lab to overcome a critical limitation of CRISPR. Specifically, the groundbreaking work provides a system to deliver the cargo required for generating the gene editing machine known as CRISPR-Cas9. The team developed a way to transform the Cas-9 protein into a spherical nucleic acid (SNA) and load it with critical components as required to access a broad range of tissue and cell types, as well as the intracellular compartments required for gene editing.
The research, published today in a paper titled, “CRISPR Spherical Nucleic Acids,” in the publication Journal of the American Chemical Society, and shows how CRISPR SNAs can be delivered across the cell membrane and into the nucleus while also retaining bioactivity and gene editing capabilities.
The work builds on a 25-year effort steered by nanotechnology pioneer Chad A. Mirkin, who led the study, to uncover the properties of SNAs and the factors that distinguish them from their well-known linear cousin, the blueprint of life. Mirkin is famed for his invention of SNAs, structures typically comprised of spherical nanoparticles densely covered with DNA or RNA, giving them chemical and physical properties radically different from those forms of nucleic acids found in nature.
Mirkin is the George B. Rathmann Professor of Chemistry in the Weinberg College of Arts and Sciences at Northwestern and director of the International Institute for Nanotechnology. Mirkin also is a professor of chemical and biological engineering, biomedical engineering and materials science and engineering in the McCormick School of Engineering and a professor of medicine at Northwestern University Feinberg School of Medicine. He is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern.
Many classes of SNAs exist, with cores and shells of different chemical compositions and sizes, and SNAs are now being evaluated as potent therapeutics in six human clinical trials, including ones for debilitating diseases like glioblastoma multiforme (brain cancer) and a variety of skin cancers.
“These novel nanostructures provide a path for researchers to broaden the scope of CRISPR utility by dramatically expanding the types of cells and tissues that the CRISPR machinery can be delivered to,” said Mirkin. “We already know SNAs provide privileged access to the skin, the brain, the eyes, the immune system, the GI track, heart and lungs. When this type of access is coupled to one of the most important innovations in biomedical science in the last quarter-century, good things will follow.”
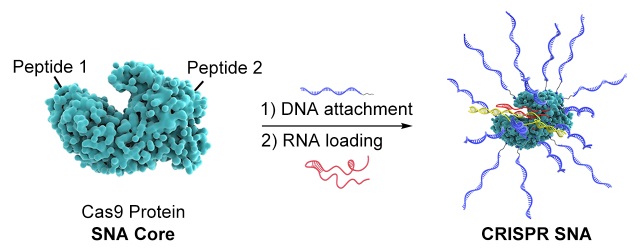
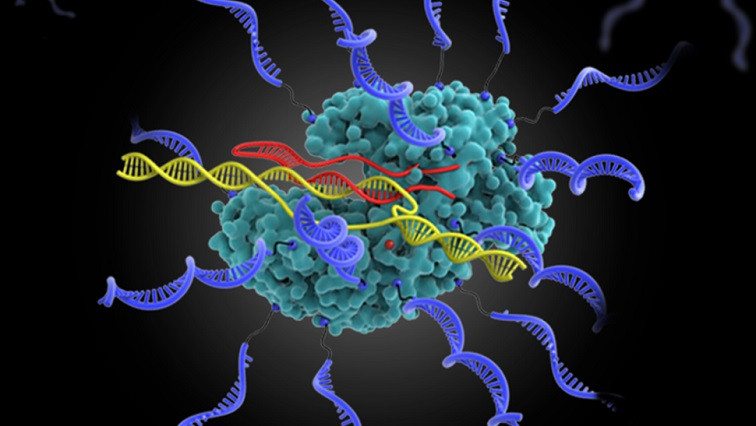
CRISPR RNP (CRISPR SNA at work: in complex with single-guide RNA (red), CRISPR SNA can recognize and cleave the gene target of interest (yellow)). The complexed form of Cas9 protein and single-guide RNA is referred to as the ribonucleoprotein (RNP).
In this current research, Mirkin’s team used Cas9, a protein needed for gene editing, as the core of the structure, and attached DNA strands to its surface to make a new type of SNA. In addition, these SNAs were preloaded with RNA capable of performing gene editing and fused with peptides to control their ability to navigate compartmental barriers of the cell, thereby maximizing efficiency. These SNAs, like other classes of them, effectively enter cells without the use of transfection agents (which are often needed to deliver genetic materials into cells) and exhibit high gene editing efficiency between 32% and 47% across several human and mouse cell lines.
Read the original article on Northwestern University.