This breakthrough has been carried out by Professor Changwook Lee and his research team in the Department of Biological Sciences at UNIST. In this study, the research team reported a new type of protein-based nanocomposite that dramatically enhances the in vivo efficacy of the TNF-related apoptosis-inducing ligand (TRAIL), a promising anticancer drug candidate known for the treatment of many cancers.
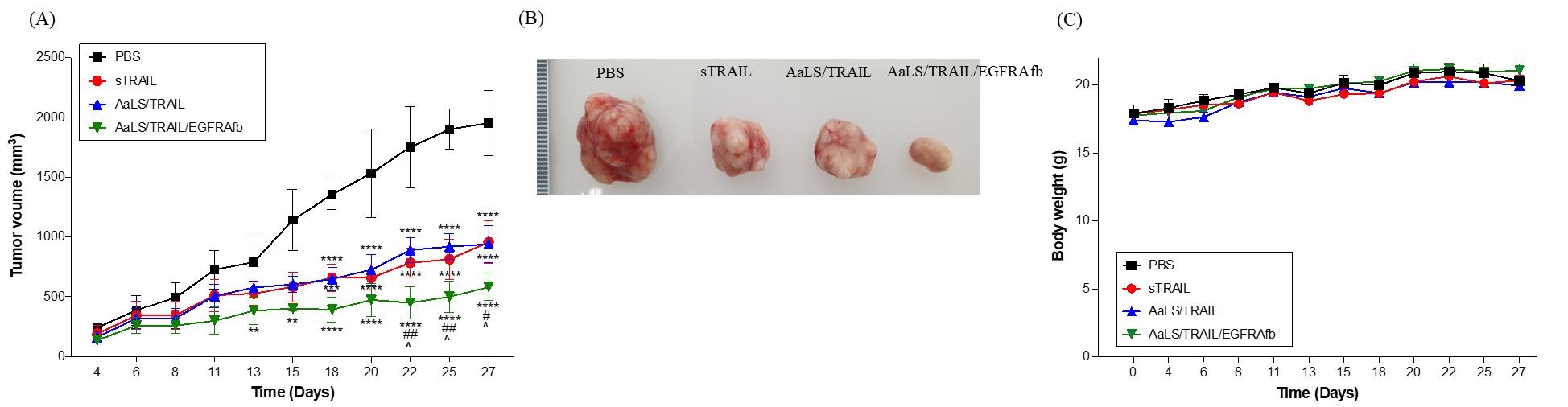
Figure 1. Schematic illustration of the construction of a polyvalent TRAIL and EGFRAfb dual-displaying AaLS that actively targets EGFR of the tumor sites and synergistically boosts apoptotic cancer cell death.
In this study, a lumazine synthase protein cage nanoparticle isolated from Aquifex aeolicus (AaLS) was used as a multiple ligand-displaying nanoplatform to display polyvalently both TRAIL and EGFR binding affibody molecules (EGFRAfb) via a SpyTag/SpyCatcher protein-ligation system, to form AaLS/TRAIL/EGFRAfb.
According to the research team, “The AaLS/TRAIL/EGFRAfb efficiently disrupted the EGF-mediated EGFR survival signaling pathway by blocking EGF/EGFR binding and strongly activating both the extrinsic and intrinsic apoptotic pathways, to maximize apoptotic cancer cell death.”
Besides, using an A431 tumor-bearing mouse model and NIR in vivo imaging, the research team also demonstrated the EGFRAfb-mediated active targeting and subsequent accumulation of AaLS/TRAIL/EGFRAfb at the tumor sites in vivo, successfully. Indeed, the A431 tumor-bearing mice treated with AaLS/TRAIL/EGFRAfb exhibited a noticeable suppression of the tumor growth without any significant side effects, according to the research team.
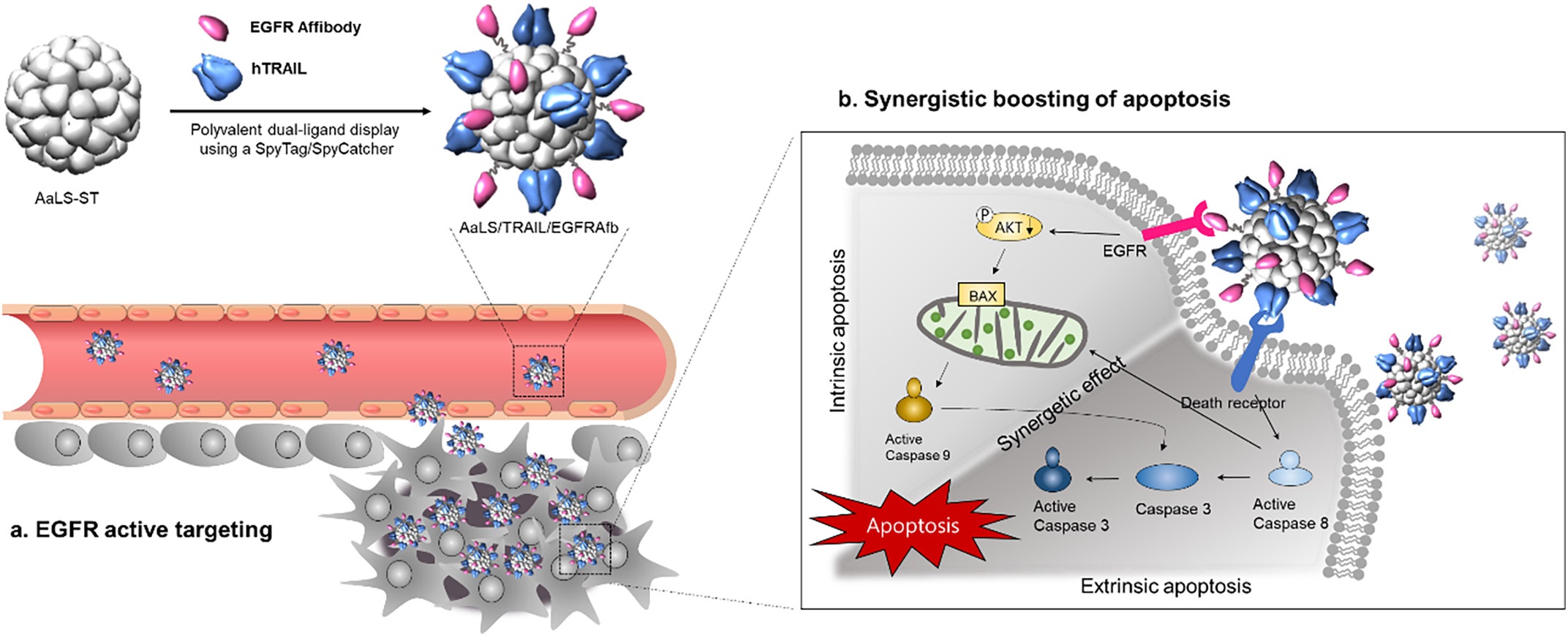
Figure 2. In vivo antitumor effect of AaLS/TRAIL/EGFRAfb. (B) Tumor sizes were measured in mice treated with PBS (black squares), sTRAIL (red circles), AaLS/TRAIL (blue triangles), or AaLS/TRAIL/EGFRAfb (green reverse triangles) every 2 or 3 days using a caliper. (C) Images of biopsied tumors from sacrificed mice treated with PBS, sTRAIL, AaLS/TRAIL, and AaLS/TRAIL/EGFRAfb. (D) Body weights of mice treated with PBS (black squares), sTRAIL (red circles), the AaLS/TRAIL (blue triangles), and AaLS/TRAIL/EGFRAfb (green reverse triangles).
“Our findings suggest that AaLS/TRAIL/EGFRAfb could be used as an effective protein-based therapeutic for EGFR-positive cancers, which are difficult to manage using mono-therapeutic approaches,” said the research team. “[T]he versatile AaLS-based nanoplatforms may offer an opportunity to develop novel therapeutic platforms for carrying multiple protein-based ligands and modulators.”
Read the original article on Ulsan National Institute of Science and Technology (UNIST).