Protein-encoding mRNA drugs find a home inside the cell, but getting them there is no small feat. To shuttle mRNA across the cell membrane and protect it from degradation by nucleases, researchers use tiny lipid nanoparticles that encapsulate the mRNA and release it inside the cell.
Administering lipid nanoparticles to the bone where mRNA can stimulate the expression of proteins that combat bone disease and injury proves equally difficult. Bones struggle to take up nanoparticles due to the blood-bone marrow barrier, reduced blood flow and vasculature compared to other organs, and low attraction to biomolecules, hindering the delivery of mRNA cargo. Methods to efficiently supply lipid nanoparticles to the bone could help launch mRNA drugs for conditions such as osteoporosis and bone cancer.
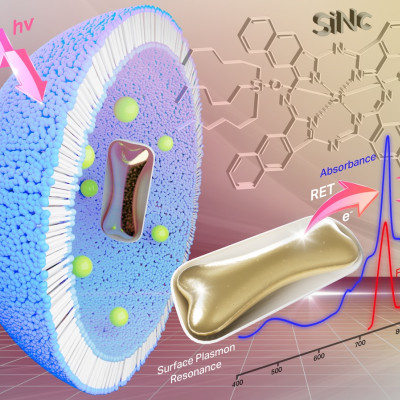
In a recent study in the Journal of the American Chemical Society, researchers at the University of Pennsylvania developed a lipid that sticks to bone minerals, increasing nanoparticle accumulation and mRNA delivery to the bone. In addition to its therapeutic potential, their work provides a new approach for directing mRNA therapeutics to evasive environments.
To help the nanoparticles cling to bone, the researchers turned to bisphosphonate. This small molecule binds to the calcium ion in hydroxyapatite, a prominent component of bone’s mineral makeup. They designed a lipid that incorporates bisphosphonate, which “kind of makes the bone act like a lint brush in that the particles can collect along it,” said Michael Mitchell, a nanoparticle bioengineer at the University of Pennsylvania and coauthor of the study.
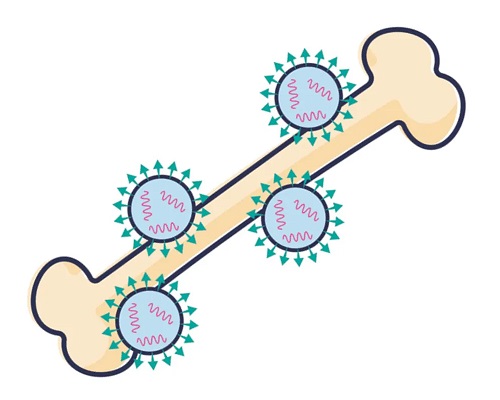
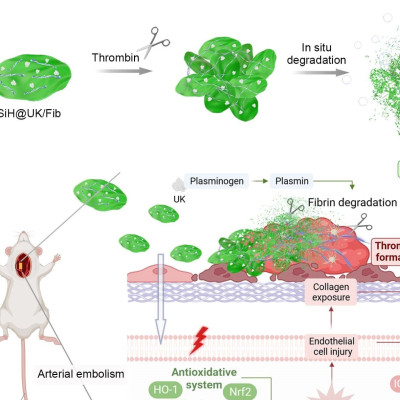
Lipid nanoparticles featuring a bone-binding bisphosphonate molecule help deliver mRNA to the bone.
The team created a series of nanoparticles from 21 unique bisphosphonate lipids and encapsulated mRNA encoding a reporter protein. In screening the nanoparticles in cells, they identified one formulation that gave higher mRNA delivery than the same particle lacking the bisphosphonate group. They also observed that this nanoparticle showed much stronger binding to hydroxyapatite compared to its bisphosphonate-free counterpart. The researchers then intravenously administered the nanoparticle to mice and found that the addition of the bisphosphonate group increased particle accumulation and protein expression in the bone.
Finally, the researchers intravenously treated mice with lipid nanoparticles carrying mRNA encoding the therapeutic growth factor BMP-2. They observed that due to its enhanced uptake in the bone, the bisphosphonate nanoparticle increased the expression of BMP-2 both on the surface of the bone and deep in the marrow relative to the standard lipid particle. The results revealed a range of possible applications for the bone-loving nanoparticles, from driving the production of regenerative proteins for fracture healing to editing genetic material in hematopoietic stem cells in bone marrow.
The study is an exciting proof-of-concept for mRNA delivery to the bone, said Blanka Sharma, a biomedical engineer at the University of Florida who was not involved in the research. As some nanoparticles gathered in the liver of the mice, off-target effects — a widespread challenge in the nanomedicine field — should be investigated, she added. “The limitation is almost always how much of what we inject systemically is actually going to where we want it to go?” she said.
The researchers plan to evaluate toxicity resulting from the particles’ biodistribution and to explore alternate administration routes. “Maybe in the near future, we can try some local injection delivery methods for reducing off-target effects,” said Lulu Xue, a postdoctoral fellow in the bioengineering department at the University of Pennsylvania and coauthor of the study.
As scientists strive to deliver lipid nanoparticles to several specific organs, Mitchell hopes that the strategy of integrating a binding group into the design of the lipid can be harnessed beyond the bone. “This type of chemistry can be used to incorporate other small molecules into lipids that could target other cells and tissues,” he said.
Whether they are optimized to target the tibia or adapted to access other parts of the body, there’s no bones about the potential of these particles.
Read the original article on Drug Discovery News.