A study conducted by pre-PhD researcher Pablo S. Valera and recently published in PNAS demonstrates the potential of surface-enhanced Raman spectroscopy (SERS) to explore metabolites secreted by cancer cells in cancer research. The study, which has been led by Ikerbasque Research Professors Luis Liz-Marzán (from CIC biomaGUNE) and Arkaitz Carracedo (of CIC bioGUNE) and in which other researchers from both centers, also members of the Networking Biomedical Research Centre (CIBER), have participated as well, provides valuable information to guide more specific experiments to reveal the function of such metabolites secreted in the tumor microenvironment or environment, which could lead to new therapeutic strategies.
The tumor microenvironment is a complex ecosystem formed by interactions between tumor and healthy cells. It is a dynamic pseudoorgan that determines the development and progression of cancers. Although attention has traditionally focused on intercellular communication mediated by protein messengers, attention has recently turned to metabolites (or small compounds) secreted by tumors into the extracellular space.
Traditional techniques for tracking these metabolites in complex cellular contexts are limited, but surface-enhanced Raman spectroscopy (SERS) has emerged as a promising alternative due to its simplicity of operation. In this study, a SERS-based strategy proposes “investigating metabolites secreted by tumor cells lacking methylthioadenosine phosphorylase (a common genetic event associated with poor prognosis in various types of cancer, such as breast cancer and glioblastoma),” explained Valera. SERS “is a spectroscopic technique that uses gold nanoparticles to detect molecules in a biofluid. It is a fairly fast technique, in which no pre-treatment of the samples is required,” he added.
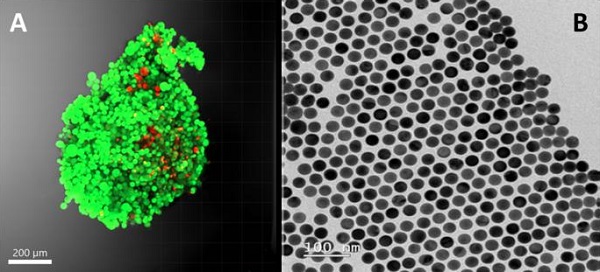
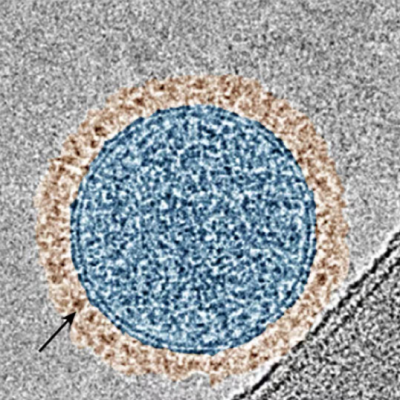
(A) Fluorescence photography of a 3D fibroblast culture (a healthy cell type) and (B) image of the gold nanoparticles used in SERS technology.
Cellular communication brought about by metabolites secreted by tumor cells
Using SERS, the researchers discovered that these cells secrete purine metabolites, which can be metabolized by healthy cells, giving rise to molecular changes consistent with cancer aggressiveness; this accounts for the reprogramming, which has never been seen previously, of the tumor environment in cancers with methylthioadenosine phosphorylase suppression: “We were able to detect this metabolite, not only in tumor cells but also in the rest of the healthy cells that are in contact with the tumor cells. So we detected that there is a relationship between tumor cells and healthy cells by means of this metabolite, and that it also brings about a change in the behavior of healthy cells so that to a certain extent they help the tumor to develop,” said Valera. It is worth pointing out that, “unravelling the complexity of such interactions in cancer patients could, in turn, pave the way for new therapeutic approaches,” he added.
The successful applying of SERS in this study demonstrates that this technology could speed up the ability to rapidly capture metabolic interactions in complex environments. In fact, the simple, rapid acquisition of signals in SERS, together with its high sensitivity, meets the requirements to be a front-line tool that can subsequently guide more specific analyses. A complete picture of the metabolic status of the tumor microenvironment can be obtained by monitoring with complementary techniques. It is also important to highlight that an effective synergy between SERS and other analytical methods has been demonstrated.
Read the original article on EurekAlert.