Since the method would target a more specific area, it would allow for a lower dosage and could provide protection against evolving viruses and multiple diseases.
With a $323,000 grant from Merck & Co. Inc., Sharan Bobbala, assistant professor in the WVU School of Pharmacy, is exploring how lipid nanoparticles can precisely deliver adjuvants — the molecules in vaccines that stimulate the immune system — to white blood cells.
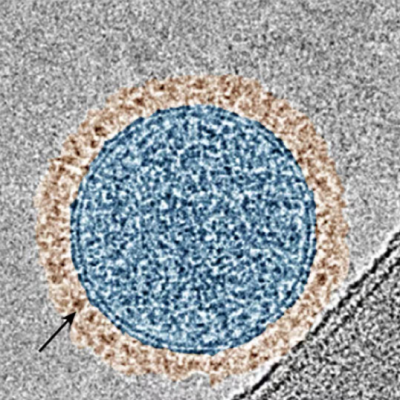
These nanoparticles are tiny spherical structures made up of fatty molecules that encapsulate drugs or genetic material and transport them to the interior of a cell. They have the unique natural ability to target immune cells.
“Intracellular delivery of adjuvants with greater precision may allow enhanced and more durable immune responses with lower vaccine doses,” Bobbala said. The ability to protect against a virus with a smaller amount of medicine would also be more cost effective and help improve global supply of vaccines.
“This research will help in achieving broadened antibody responses, which means vaccines will be effective against pathogens that show significant antigenic drift, strain variations, or both,” Bobbala said.
An antigenic drift occurs when a virus changes slightly over time to produce a new strain the immune system does not recognize.
According to the Centers for Disease Control and Prevention, that’s what causes people to get the flu more than once, and it’s the reasoning behind why the vaccines against it are reevaluated and modified every year. Certain rhinoviruses and HIV have also been known to produce an antigenic drift.
Bobbala said the nanoparticle delivery platform for his study is similar to one used for mRNA vaccines to protect people from COVID-19. No difference would occur in how the vaccine is administered into the muscle nor how it performs by tricking the immune system into reacting to what it senses as an invasion.
“This research would be beneficial in developing effective vaccines against a wide range of diseases given that the right combination of adjuvants is chosen,” he said. “It will need to be tuned for each disease based on the type of immune response required.”
For the two-year study, Bobbala will develop and optimize single- and multi-adjuvanted nanoparticle formulations and monitor their immune stimulation abilities in both animals and laboratory vessels.
“Once the most effective adjuvanted formulations are determined, the next step will be to employ them as components of prophylactic — preventive — and therapeutic vaccines against intracellular pathogens and cancers, respectively,” Bobbala said.
Read the original article on West Virginia University (WVU).