Autoimmune diseases are mysterious. It wasn’t until the 1950s that scientists realized that the immune system could harm the organs of its own body. Even today, the fundamental causes and inner workings of most autoimmune diseases remain poorly understood, limiting the treatment options for many of these conditions.
Over the past several years, however, research has found clues for how autoimmune diseases might arise. This research has shown that DNA attached to small particles within the bloodstream is a likely culprit involved in many autoimmune diseases, especially systemic lupus erythematosus, or just lupus for short, which primarily affects young women and can cause kidney damage.
However, due to the large variety in sizes of both particles and DNA in the blood, testing to what extent and under what circumstances these DNA-particle combinations play a role in disease has been extremely difficult.
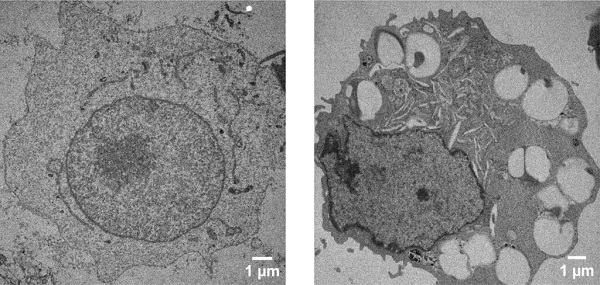
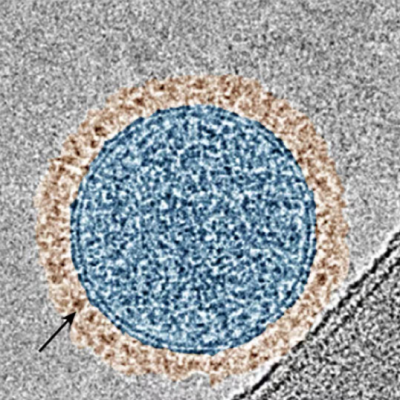
A healthy cell’s internal structure (left) compared to a cell that has taken up nanoparticles coated with DNA (right). The large cavities are the places where the nanoparticle was internalized, helping researchers better understand what cell receptors are being activated.
Researchers at Duke University have now developed a way to systematically test how these DNA-bound particles interact with the immune system. By using tiny particles of specific sizes, attaching DNA strands of certain lengths and exposing the resulting complexes to immune cells in a lab dish, the researchers show a better fundamental understanding of these diseases may be possible.
The results were published online March 5 in the journal Proceedings of the National Academy of Sciences (PNAS).
“Our approach identified the cellular pathway that causes the harmful response to these hybrid particles, and showed that DNA bound to the surfaces of nanoparticles is protected from being degraded by enzymes,” said Christine Payne, the Yoh Family Professor of Mechanical Engineering & Materials Science. “We think these are extremely important results that will form the basis for future studies with our novel system.”
While DNA is usually locked away within a cell’s nucleus, it often gets into the bloodstream when cells die or are attacked by viruses and bacteria. While most so-called “cell-free DNA” only lasts minutes before being broken down by the body, in some people and situations, it can persist for much longer. In recent work, high levels of cell-free DNA have been closely related to the severity of lupus symptoms, and many doctors are now testing ways to use it to monitor disease activity.
Cell-free DNA may escape elimination largely by forming complexes with other molecules or attaching itself to naturally occurring particles. Depending on the origin of the DNA, it can range in length from a few hundred base pairs to several thousand. And the particles it can attach to range from 100 to 1000 nanometers in diameter.
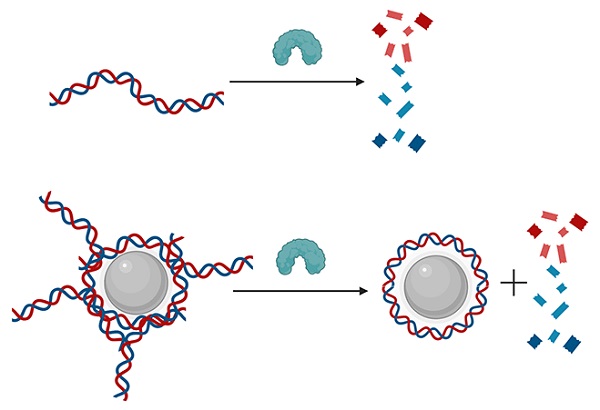
Normally, the enzyme DNase cuts cell-free DNA into tiny pieces (top). But when DNA is attached to a nanoparticle, the enzyme can only clip the ends, leaving a DNA “corona” around the nanoparticle. These complexes are believed to contribute to the symptoms of many autoimmune diseases.
“Experimenting with the particles actually found in blood is difficult because they come in so many different sizes and combinations,” said Dr. David Pisetsky, professor of medicine and integrative immunobiology at the Duke University School of Medicine. “Where previous work has focused on using nanoparticles for therapy, here we’re exploring using participles to understand disease mechanisms, which can be very informative for important medical questions.”
Payne worked with members of her laboratory to fabricate tightly controlled synthetic particles at both ends of the naturally occurring size spectrum. They then attached DNA strands from E. Coli either a few hundred base pairs long or 10,000 base pairs long to particles both large and small.
With a wide range of synthetic DNA-particle complexes in hand, they mixed various combinations with human macrophages, a type of white blood cell that surrounds and kills microorganisms, removes dead cells, and stimulates the action of other immune cells.
“I joined the lab over a year ago and have been working on characterizing the nanoparticle coronas to understand their size, amount of DNA and how the DNA degrades,” said Diego Montoya, a third-year undergraduate student working in Payne’s lab and a co-author of the paper. “It’s been a lot of fun and a privilege working with everyone on this research.”
The first important observation the team made was that DNA attached to nanoparticles was protected from degrative enzymes, and that larger nanoparticles provided more protection.
“We think the enzymes might not be able to access the DNA to destroy it because of the shape the DNA makes with the surface of the nanoparticle,” said Faisal Anees, a PhD student in Payne’s lab. “But there might be other effects going on, so that’s a question we’re trying to answer more definitively now.”
The results showed that the macrophages responded to all types of DNA-particle complexes by producing inflammatory signals for other cells to follow, a hallmark of many autoimmune diseases. They also demonstrated that this response is created through a specific signaling pathway called cGAS-STING.
The researchers stress that the combined results do not yet provide a smoking gun for the cause of lupus or other autoimmune diseases, which are likely varied and nuanced.
“All the ways the immune system attacks itself are really complex, hard to understand and difficult to treat,” Payne said. “This approach gives researchers a way to drill down and pinpoint factors that they wouldn’t be able to with a purely biological system.”
“We now have a well-defined model system that gives us the ability to ask these questions about causation versus correlation,” added Pisetsky, who has been researching autoimmune diseases for almost half a century. “It also gives us a new method for exploring potential therapies.”
Read the original article on Duke University.