“As complex living systems, we likely have trillions upon trillions of tiny nanoscopic holes in our cells that facilitate and regulate the crucial processes that keep us alive and make up who are,” says Marija Drndić, a physicist at the University of Pennsylvania who develops synthetic versions of the biological pores that “guide the exchange of ions and molecules throughout the body.”
The ability to control and monitor the flow of molecules through these pores has opened new avenues for research in the last two decades, according to Drndić, and the field of synthetic nanopores, where materials like graphene and silicon are drilled with tiny holes, has already led to significant advances in DNA sequencing.
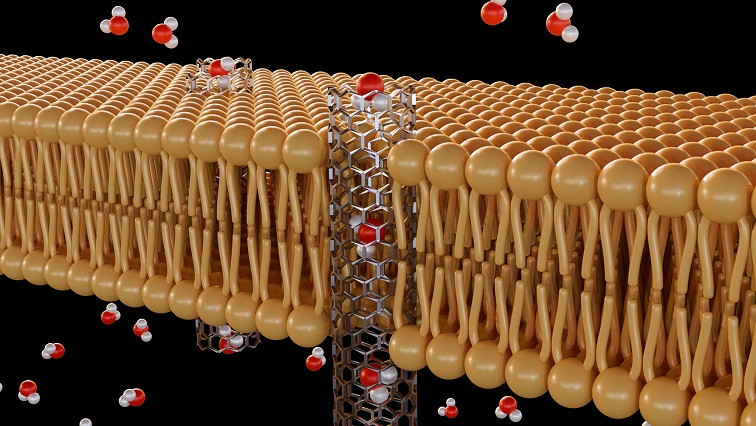
In a paper published in Nature Nanotechnology, Drndić and Dimitri Monos, her longtime collaborator at the Perelman School of Medicine and Children’s Hospital of Philadelphia (CHOP), presented a new kind of nanopore technology with the development of a dual-layer nanopore system: a design that consists of two or more nanopores, stacked just nanometers apart, which allows for more precise detection and control of molecules like DNA as they pass through.
“With current platforms, when molecules like DNA are placed on the nanopores it’s sort of like spaghetti in a pot: tangled and difficult to work with, let alone guide through a single hole,” Monos says. Typically, researchers need to use proteins to unwind and straighten it, which, while effective, has many limitations due to degradation that leads to reduced sensitivity and shorter operational lifespans, he says.
“But with this new design,” Monos says, “we’re essentially guiding molecules through two coupled nanopores in the material, providing a controlled, smoother passage of molecules and making them easier to detect and analyze.”
The researchers call this new platform GURU to denote that “it’s a guided and reusable,” way to study molecules, which confers a few key benefits, most notably, the ability to better assess the length and conformation of molecules as they pass through the nanopores.
“Because we know the precise distance between the two nanopores, we can use it as a kind of ruler,” Drndić says, “comparing the signals to determine the exact length of the DNA passing through.” This system offers researchers an unprecedented level of control, enabling them to measure molecular properties more accurately than ever before.
This increased resolution provides a clearer picture of the shape and structure of molecules, including DNA, RNA, and proteins.
Unlike traditional single-pore systems, GURU allows for longer times in the sensing zone, which enhances the detection process, and one of the most intriguing outcomes of this slowing down is the discovery of unique signal patterns shaped like the letters “W” and “T,” a characteristic that the postdoctoral researcher and first author of the paper, Chih-Yuan (Scottie) Lin, first observed.
“These patterns correspond to the way molecules interact with the dual-pore system,” Lin says. “When we measure signals that look like a ‘W’, it’s because the molecule is engaging with both the bottom and top nanopores in sequence, reflecting how it enters the lower pore, exits slightly, and then reengages with the upper layer.”
This pattern provides the researchers with detailed information about the molecule’s passage through the system, revealing its interactions with each layer.
The T-shaped signal, Lin says, occurs when a molecule is long enough to block both nanopores simultaneously, giving a clear indication of its full length. “These signals provide us with real-time data on the length and position of the molecule.”
As the teams continue to refine their system, they believe it could lead to more efficient, accurate, and cost-effective sequencing technologies that overcome the limitations of current protein-based nanopore systems.
“What really cemented our collaboration was the shared goal of improving sequencing technology, particularly for applications like human leukocytic antigen (HLA) genes that require long DNA reads,” Monos says. As the director of the Immunogenetics Laboratory at CHOP, he works extensively with HLA genes, which are crucial for immune system compatibility in organ transplantation.
“HLA genes are among the most complex regions of the human genome, and accurate long-read sequencing is essential to understanding their variations,” Monos says. “That’s where nanopore technology like GURU comes in; it offers the potential for more precise and comprehensive sequencing in this challenging area.”
By eliminating the need for proteins and creating a purely solid-state system, their joint efforts have yielded a platform that not only advances the field of nanopore technology but also opens new possibilities for clinical applications.
“The problems we’re trying to solve with nanopores, like DNA sequencing and molecular detection, require expertise from people all over the place,” Drndić says, “it’s not just about the physics or materials science. We need input from biologists to understand the molecules, chemists to help with the reactions, and medical professionals to see the real-world applications.”
Read the original article on University of Pennsylvania.