The event occurred as part of a new Northwestern study, during which scientists sought to understand how palladium, a rare metallic element, catalyzes the gaseous reaction to generate water. By witnessing the reaction at the nanoscale, the team unraveled how the process occurs and even uncovered new strategies to accelerate it.
Because the reaction does not require extreme conditions, the researchers say it could be harnessed as a practical solution for rapidly generating water in arid environments, including on other planets.
“Think of Matt Damon’s character, Mark Watney, in the movie ‘The Martian,’” said study senior author Vinayak Dravid. “He burned rocket fuel to extract hydrogen and then added oxygen from his oxygenator. Our process is analogous, except we bypass the need for fire and other extreme conditions. We simply mixed palladium and gases together.”
Dravid is the Abraham Harris Professor of Materials Science and Engineering at Northwestern’s McCormick School of Engineering and founding director of the Northwestern University Atomic and Nanoscale Characterization Experimental (NUANCE) Center, where the study was conducted. He also is director of global initiatives at the International Institute for Nanotechnology.
New technology enabled discovery
Since the early 1900s, researchers have known that palladium can act as a catalyst to rapidly generate water. But how, exactly, this reaction occurs has remained a mystery.
“It’s a known phenomenon, but it was never fully understood,” said Yukun Liu, the study’s first author and a Ph.D. candidate in Dravid’s laboratory. “Because you really need to be able to combine the direct visualization of water generation and the structure analysis at the atomic scale in order to figure out what’s happening.”
But viewing the process with atomic precision was simply impossible — until nine months ago. In January 2024, Dravid’s team unveiled a novel method, previously published in Science Advances, to analyze gas molecules in real time. Dravid and his team developed an ultra-thin glassy membrane that holds gas molecules within honeycomb-shaped nanoreactors, so they can be viewed within high-vacuum transmission electron microscopes.
“Using the ultrathin membrane, we are getting more information from the sample itself,” said Kunmo Koo, first author of the Science Advances paper and a research associate at the NUANCE Center, where he is mentored by research associate professor Xiaobing Hu.
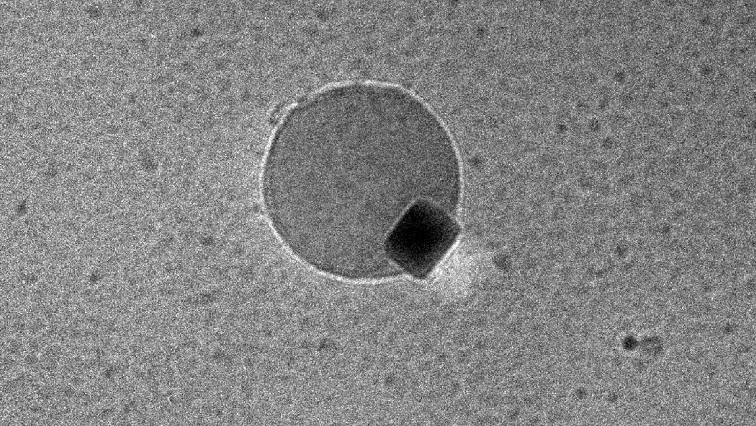
Smallest bubble ever seen
Using the new technology, Dravid, Liu and Koo examined the palladium reaction. First, they saw the hydrogen atoms enter the palladium, expanding its square lattice. But when they saw tiny water bubbles form at the palladium surface, the researchers couldn’t believe their eyes.
“We think it might be the smallest bubble ever formed that has been viewed directly,” Liu said. “It’s not what we were expecting. Luckily, we were recording it, so we could prove to other people that we weren’t crazy.”
Recipe for optimization
After confirming the palladium reaction generated water, the researchers next sought to optimize the process, discovering that adding hydrogen first, followed by oxygen, led to the fastest reaction rate. Because hydrogen atoms are so small, they can squeeze between palladium’s atoms — causing the metal to expand.
After filling the palladium with hydrogen, the researchers added oxygen gas. The hydrogen came out of the palladium to react with the oxygen, and the palladium shrunk back to its initial state.
Sustainable system for deep space
Although the study, which was published in the Proceedings of the National Academy of Sciences, focused on studying bubble generation at nanoscale, larger sheets of palladium would generate much larger quantities of water. The team imagines that others, in the future, potentially could prepare hydrogen-filled palladium before traveling into space. Then, to generate water for drinking or for watering plants, travelers will only need to add oxygen.
“Palladium might seem expensive, but it’s recyclable,” Liu said. “Our process doesn’t consume it. The only thing consumed is gas, and hydrogen is the most abundant gas in the universe. After the reaction, we can reuse the palladium platform over and over.”
Read the original article on Northwestern University.