The method, described in Nature Biomedical Engineering, mirrors the iterative process of developing a culinary dish and may lead to safer, more effective mRNA vaccines and therapeutics.
Just as a chef perfects a dish by experimenting with flavors and textures, the researchers used an iterative process, testing variations to find the ideal structure for the ionizable lipid. This lipid’s structure influences the ability of LNPs to successfully deliver their contents and advances mRNA therapies for vaccines and gene editing.
A Breakthrough in LNP Design
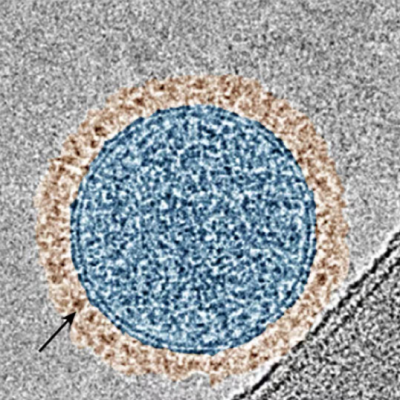
Nanoparticles have transformed how mRNA vaccines and therapeutics are delivered by allowing them to travel safely through the body, reach target cells and release their contents efficiently. On its own, RNA is fragile, and would otherwise dissolve without ever reaching its intended target.
At the heart of these nanoparticles are ionizable lipids, special molecules that can switch between charged and neutral states depending on their surroundings. This switch is essential for the nanoparticle’s journey: In the bloodstream, ionizable lipids stay neutral, preventing toxicity. But once inside the target cell, they become positively charged, triggering the release of the mRNA payload.
Led by Michael J. Mitchell, Associate Professor in Bioengineering, the researchers refined this delivery process by optimizing the structure of ionizable lipids. Moving beyond existing methods limited by tradeoffs between speed and accuracy, the team developed a step-by-step, “directed chemical evolution” process. Through five cycles, with each further refining the lipids, they created dozens of high-performing, biodegradable lipids — some even surpassing industry standards.
The Secret Sauce: Directed Chemical Evolution
To develop safer, more effective ionizable lipids, the Penn Engineers employed a unique approach that combines two prevailing methods: medicinal chemistry, which involves slowly and laboriously designing molecules one step at a time, and combinatorial chemistry, which involves generating many different molecules quickly through simple reactions. The former has high accuracy but low speed, while the latter has low accuracy and high speed.
“We thought it might be possible to achieve the best of both worlds,” says Xuexiang Han, the paper’s first author and, until recently, a postdoctoral fellow in the Mitchell Lab. “High speed and high accuracy, but we had to think outside the traditional confines of the field.”
By borrowing the idea of directed evolution, a technique used in both chemistry and biology that mimics the process of natural selection, the researchers combined precision with rapid output to achieve their ideal lipid “recipe.”
The process begins with the generation of a wide variety of molecules, which are screened for their ability to deliver mRNA. The best-performing lipids are then used as starting points for generating another round of molecular variants, and so on, until only high-performing variants remain.
An Innovative Ingredient: A3 Coupling
A crucial contributor to the team’s recipe for improved ionizable lipids is A3 coupling, a three-component reaction named for its chemical ingredients: an amine, an aldehyde and an alkyne.
The reaction, which has never been leveraged to synthesize ionizable lipids for LNPs, uses inexpensive, commercially available ingredients and produces only water as a byproduct, making it a cost-effective and environmentally friendly choice for rapidly producing the large numbers of ionizable lipid variants needed as ingredients for directed evolution.
“We found that the A3 reaction was not only efficient, but also flexible enough to allow for precise control over the lipids’ molecular structure,” says Mitchell. This flexibility was key to fine-tuning the ionizable lipid properties for safe and effective mRNA delivery.
Why This Advance Matters
This new method for designing ionizable lipids is expected to have broad implications for mRNA-based vaccines and therapeutics, which are poised to treat a range of conditions, from genetic disorders to infectious diseases.
In this work, the optimized lipids improved mRNA delivery in preclinical models for two high-priority applications: editing genes that cause hereditary amyloidosis, a rare disease that results in abnormal protein deposits throughout the body, and improving delivery of the COVID-19 mRNA vaccine. In both cases, the engineered lipids showed higher performance than current industry-standard lipids.
Beyond these specific applications, the new approach has the potential to accelerate the development of mRNA therapies overall. While it can take years to develop an effective lipid using traditional methods, the team’s directed evolution process could reduce this timeline to just months or even weeks.
“Our hope is that this method will accelerate the pipeline for mRNA therapeutics and vaccines, bringing new treatments to patients faster than ever before,” says Mitchell.
A New Frontier for mRNA Delivery
LNPs represent a safe, flexible way to deliver genetic material, but their success hinges on the properties of their ionizable lipids. The Penn Engineers’ iterative design process allows researchers to improve these lipids with unprecedented speed and precision, bringing the next generation of mRNA therapies closer to reality.
With this innovative recipe for LNPs, Penn Engineers have taken a major step forward in advancing mRNA technology, offering hope for a faster and more efficient path to life-changing treatments.
Read the original article on University of Pennsylvania.