The manuscript, accepted in ACS Nano on Aug. 18, is titled "Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Host Cell Surface Angiotensin Converting Enzyme 2 Binding and Endocytosis." SARS-CoV-2 is known to attach to angiotensin converting enzyme 2 (ACE2) receptors via its external 'Spike' proteins, which cover the surface, which the virus uses to bind with and enter human cells.
"We developed nanoparticle-based pseudo-virions that bind to the host cell and track themselves inside of cells without being contagious," said Eunkeu Oh, Ph.D., an NRL biophysicist. "This opens an opportunity to expand the same strategy to various other infectious diseases."
Together with researchers at NCATS, part of the National Institutes of Health (NIH), they worked to develop non-infectious probes to study SARS-CoV-2, which is the causative virus of the current COVID-19 pandemic.
"Simply put, keeping the virus out of cells prevents it from replicating, propagating, and exacerbating infection," said Mason Wolak, Ph.D., acting head of NRL's Optical Nanomaterials Section. "The ultimate goals of the collaboration are to clarify the fundamental mechanisms by which SARS-CoV-2 causes infection and to screen and identify potential drugs to inhibit these mechanisms."
One thing that fascinated the scientists was the potency of the quantum dot nanoparticles to induce translocation of ACE2 from the cell membrane to the interior of the cell through a process called endocytosis. Kirill Gorshkov, Ph.D., an NCATS research scientist, said experiments to block endocytosis prevented internalization of the quantum dot pseudovirus and ACE2 receptor complex.
"While we had some idea that this was possible, the extent to which this occurred with very low amounts of quantum dot pseudo-virions suggested we had a powerful system to track viral attachment and effects on the cell in real time, since these quantum dots are fluorescent," said Gorshkov.
The nanoparticle probes developed at NRL are comprised of multiple Spike protein subunits attached to the surface of a light-emitting quantum dot core.
"We refer to these probes as 'Pseudo-virions' because they approximate the shape of the SARS-CoV-2 virus while also mimicking their physiological interactions with human cells," said Wolak. "Light emitted from the Pseudo-virions allows spatiotemporal tracking of their interaction with human cells."
Wolak went on to say that binding to ACE2 is seen as the initial step of the virus towards cellular infection. Successful inhibition of this binding is an extremely high priority in the search for therapeutic interventions to mitigate the impact of the pandemic on military and public health.
"Protecting the health of deployed warfighters is of the utmost importance to ensure mission readiness and maintain fleet readiness," said Wolak. "Navy ships represent the ultimate 'closed system' - warfighters share close quarters where social distancing is nearly impossible to realistically enforce. This results in conditions that are ripe for rapid and extensive outbreaks, such as the one reported on the USS Theodore Roosevelt, which threaten mission success."
NRL and NCATS are currently designing and testing the feasibility of high-throughput cellular imaging tests to screen entire libraries of therapeutic agents for inhibition of Spike/ACE2 binding and internalization.
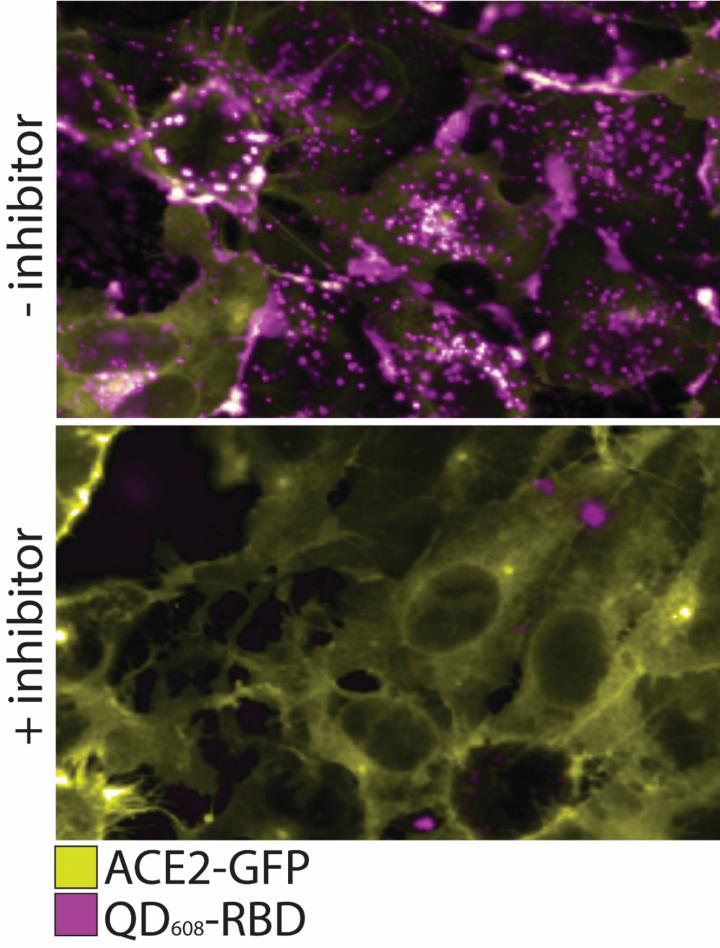
Here we see the QD608-RBD binds ACE2 and induces endocytosis. In this figure, the top panel shows ACE2-GFP (yellow) expressing cells binding and internalizing QD608-RBD (magenta). In the bottom panel, an inhibitor is added to prevent binding of QD608-RBD to ACE2-GFP, and the presence of ACE2-GFP on the cell surface is strong with little to no QD608-RBD visible.
"This also opened the door for high-throughput testing of new drugs uncovered by the other approaches at NCATS, to cross-validate the compounds with our system, and to test completely new therapeutic modalities developed at NCATS and elsewhere both biochemically at NRL, and in cell-based assays at NCATS," said Gorshkov.
These tests, carried out at NCATS, would allow for screening of up to 1,536 drug targets per experiment.
"Our team at NRL brings world-class expertise in the field of quantum dot/nanoparticles for biological applications," said Oh. "Merging our resources with the NCATS team's expertise resulted in research that couldn't be done by one team on its own."
Further research is planned to investigate the mechanism that increases infectiousness of SARS-CoV-2 with mutated Spike proteins. The NRL/NCATS team also plans to explore the possibility of using the pseudo-virions for site-specific intracellular drug delivery for purposes of disrupting SARS-CoV-2 replication mechanisms.
Wolak said, "It is very important to note that the Spike-containing pseudo-virion has potential for broad application of intracellular drug delivery to combat not only COVID19, but any disease that targets cells with ACE2 receptors."
Gorshkov said, he hopes the research excites and stimulates the next generation of future researchers to embark on a journey of exploration.
"I hope this story serves to engage young minds into the realm of what is possible when people work together, both scientifically and otherwise," said Gorshkov. "By forging these strong relationships, we can synergize our efforts and come to novel solutions from completely different perspectives and viewpoints."
Read the original article on U.S. Naval Research Laboratory.