The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the coronavirus disease (COVID-19), continues to ravage across the globe. To date, more than 64 million people have been infected with the virus worldwide.
Currently, there is no effective drug for COVID-19 or vaccine against SARS-CoV-2. Scientists are racing to shed light on the virus’s characteristics and mechanism of infection, which can help in formulating therapeutics and vaccines.
A team of researchers at the University of Manitoba in Canada said that nanoparticle targeting of autophagy at the sites could be a potent tool to effectively overcome COVID-19 while preventing the common adverse effects of the drug.
COVID-19 background
More is still needed to have a better understanding of the coronavirus pandemic. The outbreak first emerged in a seafood market in Wuhan City, China, in late December 2019. From there, it has reached over 191 countries and territories and claimed the lives of nearly 1.5 million individuals.
Scientists are working hard to determine how the virus infects humans and what mechanisms contribute to the infectivity and virulence of SARS-CoV-2. Understanding these can help in the fight against COVID-19, and at the same time, aid in the development of medicines for the illness.
What is autophagy?
Autophagy, which means “self-eating,” is the body’s way of cleaning out damaged or dead cells to regenerate new and healthier ones.
Viruses recruit cellular machinery and pathways, including autophagy, for their replication and spread. Autophagy is a cell stress response that aids in the quality control mechanism for cells by removing malfunctioning proteins, damaged organelles, and invasive microbes.
Many viruses have shown the hijacking of cellular autophagy mechanisms.
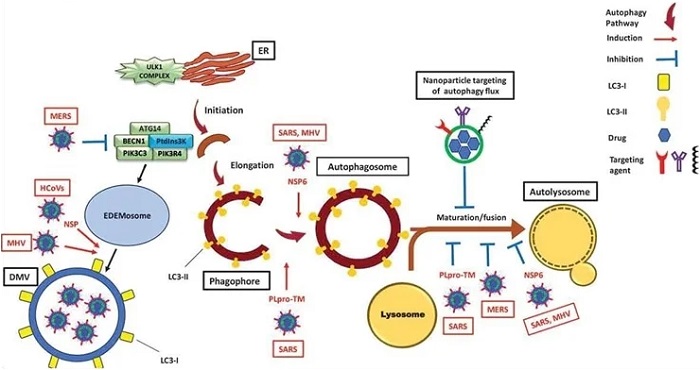
Modulation of the autophagy pathway by coronaviruses and proposal of novel smart drug-loaded nanoparticles to target this pathway to combat COVID-19. Schematic shows how coronaviruses interact with autophagy. The NSP6 protein of SARS and MHV induces the formation of autophagosomes but confines their expansion and blocks their maturation into autolysosomes. A similar effect is observed by PLpro-TM of SARS. Human CoVs (HCoVs) via their NSPs, and MHV induce the formation of LC3-I-coated DMVs needed for viral RNA transcription and replication. MERS decreases the level of BECN1 (beclin 1) and blocks fusion of autophagosomes with lysosomes. Chloroquine/hydroxychloroquine, emtricitabine/tenofovir, interferon alfa-2b, lopinavir/ritonavir and ruxolitinib, which are all under clinical trial for treatment of SARS-CoV-2, induce autophagosome accumulation by blocking their maturation into autolysosomes. Thus, designing nanoparticles for the targeted delivery of these drug to avoid their off-target effects will provide safe and effective powerful tools to combat COVID-19. ATG14: autophagy related 14; DMV: double-membrane vesicles; EDEMosome: LC3-I-positive endoplasmic reticulum-derived vesicles exporting short-lived ERAD regulators; ER: endoplasmic reticulum; LC3-I: processed MAP1LC3; LC3-II: lipidated MAP1LC3; MERS: Middle East respiratory syndrome; MHV: murine gammaherpes virus; NSP6: non-structural protein 6; PIK3C3/VPS34: phosphatidylinositol 3-kinase catalytic subunit type 3; PIK3R4/VPS15: phosphoinositide-3-kinase regulatory subunit 4; PtdIns3 K: class III phosphatidylinositol 3-kinase; PLpro-TM: membrane-anchored papain-like protease; SARS: severe acute respiratory syndrome; ULK1 complex: unc-51 like autophagy activating kinase 1.
COVID-19 treatment
At present, there is still no approved treatment for COVID-19. However, many clinicians and health experts resorted to the repurposing of approved drugs to improve the condition of people infected with SARS-CoV-2.
Drug repurposing has been associated with promising outcomes and resulted in the ongoing clinical trials for 12 drugs for COVID-19. Many of these drugs are autophagy modulators.
Interestingly, almost all of these autophagy modulators do not appear to act by directly antagonizing the effects of beta coronaviruses.
“We suggest that the beneficial effect of these drugs is possibly due to the over-accumulation of autophagosomes that can potentially induce apoptotic cell death of virally infected cells and disrupt the virus replication cycle, similar to what we observed in our recent study,” the researchers explained.
The study published in the journal Virulence highlights the potential of using novel smart drug-loaded nanoparticles to target the autophagy pathway by coronaviruses to combat COVID-19.
Autophagy modulator
To have an effective and safe autophagy modulator as a treatment for COVID-19, a carrier is needed to deliver the drug to the infected cells using nanoparticles.
Nanotechnology has come a long way and has helped various sectors in the community. Scientists can use nanoparticles for targeting active sites and preventing off-target accumulation.
Further, nanoparticles have unique characteristics, which can affect their bioavailability and circulation time. They can cross biological barriers and improve the bioavailability of poorly soluble drugs.
“Recently, nanoparticles were shown to modulate autophagy and have been exploited for overcoming obstacles encountered with autophagy modulators,” the team explained.
Also, nanoparticle-based produces are approved and under investigation for the treatment of viral infections. Hence, nanotechnology has great potential for contributing to the fight against COVID-19.
“We strongly recommend cross-disciplinary collaborations between autophagy and nanotechnology communities to accelerate the discovery of potential drug candidates and the translation of these discoveries into clinically-approved COVID-19 therapies that are both effective and safe,” the team concluded.
Read the original article on Medical News.