Led by Prof. Timothy Noël and in cooperation with the British company Vapourtec, a continuous-flow system is presented that combines a micro-flow photoreactor with a nanofiltration device for photocatalyst recycling.
Photocatalysis makes it possible to drive chemical conversions directly by sunlight, or by LED light powered by renewable electricity. As such, it presents an opportunity to render the chemical industry more sustainable and less dependent on fossil resources. However, the cost of a photocatalyst can be quite high, which often hampers industrial interest in photocatalysis.
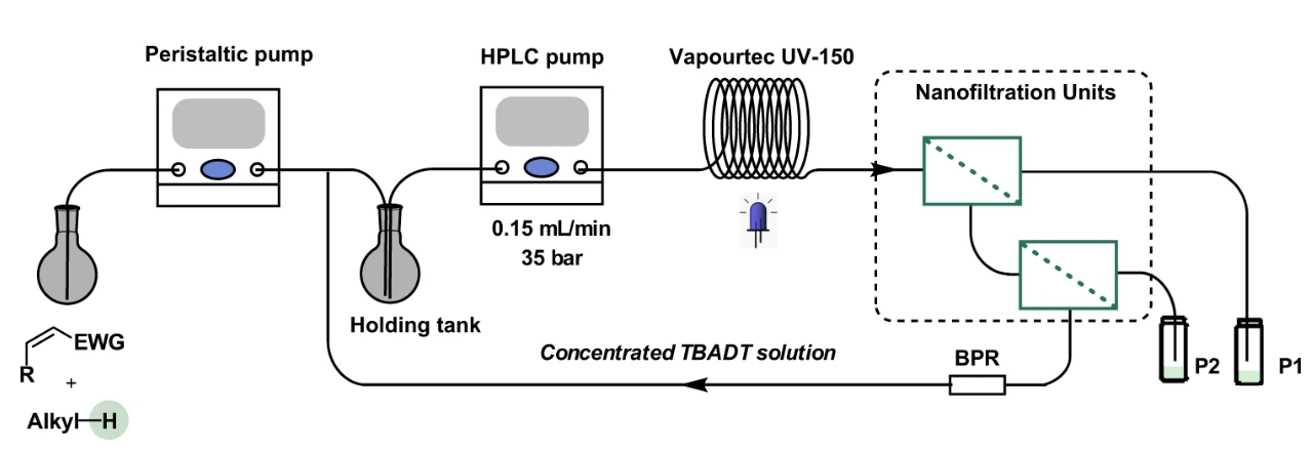
Flowchart of the multi-stage nanofiltration-based continuous-flow system employed for the recovery of TBADT. (EWG: electron withdrawing group, BPR: backpressure regulator.)
At the University of Amsterdam’s Van ‘t Hoff Institute for Molecular Sciences (HIMS), the Noël Research Group develops flow chemistry systems that help materialize the promises of photocatalysis. In Nature Communications they now describe how they have combined the flow chemistry approach with the use of nanofiltration for in-process catalyst recycling. Since this renders the cost of the catalyst essentially irrelevant, this presents an important step in bringing photocatalysis to industrial application.
In earlier research, the group developed a flow reactor that employed a decatungstate photocatalytist (TBADT) for so-called hydrogen atom transfer (HAT) processes. Combining this with the nanofiltration system, they were able to achieve a catalyst recycling percentage of over 99% in various HAT reactions (such as photocatalytic C(sp3)–H alkylation and amination). They report turnover numbers (TONs) of well over 8000, which according to Prof Noël is a record number for photocatalysts at least in his lab; it might even be the highest TON for any synthetic photocatalysis experiment. He considers the newly developed process an important stepping stone toward sustainable ‘real life’ (i.e. process-ready) photocatalysis.
Read the original article on University of Amsterdam.