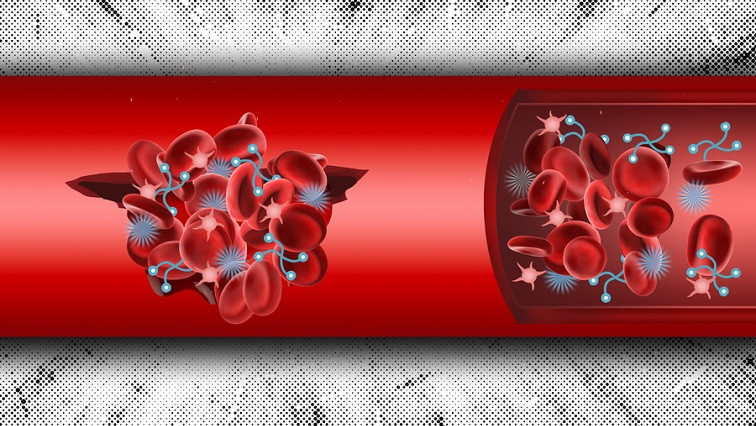
MIT engineers have designed a two-component system that can be injected into the body and help form blood clots at the sites of internal injury. These materials, which mimic the way that the body naturally forms clots, could offer a way to keep people with severe internal injuries alive until they can reach a hospital.
In a mouse model of internal injury, the researchers showed that these components — a nanoparticle and a polymer — performed significantly better than hemostatic nanoparticles that were developed earlier.
“What was especially remarkable about these results was the level of recovery from severe injury we saw in the animal studies. By introducing two complementary systems in sequence it is possible to get a much stronger clot,” says Paula Hammond, an MIT Institute Professor, the head of MIT’s Department of Chemical Engineering, a member of the Koch Institute for Integrative Cancer Research, and one of the senior authors of a paper on the study.
Unlike previously developed hemostatic systems, the new MIT technology mimics the actions of both platelets — the cells that initiate blood clotting — and fibrinogen, a protein that helps forms clots.
“The idea of using two components allows selective gelation of the hemostatic system as the concentration is enhanced in the wound, mimicking the end effect of the natural clotting cascade,” says Bradley Olsen, the Alexander and I. Michael Kasser Professor of Chemical Engineering at MIT and a senior author of the study.
MIT postdoc Celestine Hong PhD ’22 is the lead author of the paper, which appears in Advanced Healthcare Materials. Other authors of the paper include postdoc Yanpu He, undergraduate student Porter Bowen, and Professor Angela Belcher, who is head of MIT’s Department of Biological Engineering.
Artificial clotting
Blood loss from traumatic events such as car crashes contributes to more than 2.5 million deaths per year worldwide. This kind of blunt trauma can cause internal bleeding from organs such as the liver, which is difficult to detect and treat. In such cases, it’s critical to stop the bleeding as soon as possible, until a patient can be transported to the hospital for further treatment. Finding ways to prevent internal bleeding could have an especially significant impact in the armed services, where delayed treatment for internal hemorrhage is one of the largest causes of preventable death, Olsen says.
When internal injuries occur, platelets are attracted to the site and initiate the blood clotting cascade, which eventually forms a sticky plug of platelets and clotting proteins, including fibrinogen. However, if patients are losing a lot of blood, they don’t have enough platelets or fibrinogen to form clots. The MIT team wanted to create an artificial system that could help save people’s lives by replacing both of those clotting components.
“What researchers in this area have been doing in the past is trying to either recapture the therapeutic effects of platelets or recapture the function of fibrinogen,” Hong says. “What we are trying to do in this project is to capture the way they interact with each other.”
To achieve that, the researchers created a system with two types of materials: a nanoparticle that recruits platelets and a polymer that mimics fibrinogen.
For the platelet-recruiting particles, the researchers used particles similar to those they reported in a 2022 study. These particles are made of a biocompatible polymer called PEG-PLGA, which are functionalized with a peptide called GRGDS that allows them to bind to activated platelets. Because platelets are drawn to the site of an injury, these particles also tend to accumulate at injury sites.
In that 2022 study, the researchers found that when these targeting particles were in an optimal size range of 140 to 220 nanometers, they would build up at a wound site but not accumulate significantly in organs such as the lungs, where clot formation would be risky to the patient.
For this paper, the researchers modified those particles by adding a chemical group that would react with a tag placed on the second component in the system, which they call the crosslinker. Those crosslinkers, made of either PEG or PEG-PLGA, bind to the targeting particles that have accumulated at a wound site and form clumps that mimic blood clots.
“The idea is that with both of these components circulating inside the bloodstream, if there is a wound site, the targeting component will start accumulating at the wound site and also bind the crosslinker,” Hong says. “When both components are at high concentration, you get more cross-linking, and they begin forming that glue and helping the clotting process.”
Stopping the bleeding
To test the system, the researchers used a mouse model of internal injury. They found that after being injected into the body, the two-component system was highly effective at stopping bleeding, and it worked about twice as well as the targeting particle on its own.
Another important advantage of the clots is that they don’t degrade as fast as naturally occurring clots do. When patients lose a lot of blood, they are usually given saline intravenously to keep up their blood pressure, but this saline also dilutes the existing platelets and fibrinogen, leading to weaker clots and faster degradation. However, the artificial clots are not as susceptible to this kind of degradation, the researchers found.
The researchers also found that their nanoparticles did not induce any significant immune reaction in the mice compared to a glucose control. They now plan to test the system in a larger animal model, working with researchers at Massachusetts General Hospital.
In the longer term, the researchers also hope to explore the possibility of using portable imaging devices to visualize the injected nanoparticles after they have entered the body. This could help doctors or emergency medical responders quickly determine the site of internal bleeding, which currently can only be done at a hospital with MRI, ultrasound, or surgery.
“There can be hours of delay in figuring out where the source of the bleeding is, and that requires a lot of steps before the bleeding site can be treated. So, being able to combine this system with diagnostic tools is one area that we're interested in,” Hong says.
Read the original article on Massachusetts Institute of Technology (MIT).