An interdisciplinary research team from Bochum, Duisburg and Zurich has developed a new approach to construct modular optical sensors which are capable of detecting viruses and bacteria. For this purpose, the researchers used fluorescent carbon nanotubes with a novel type of DNA anchors that act as molecular handles. The anchor structures can be used to conjugate biological recognition units such as antibodies aptamers to the nanotubes. The recognition unit can subsequently interact with bacterial or viral molecules to the nanotubes. These interactions effect the fluorescence of the nanotubes and increase or decrease their brightness.
A team consisting of Professor Sebastian Kruss, Justus Metternich and four co-workers from Ruhr University Bochum (Germany), the Fraunhofer Institute for Microelectronic Circuits and Systems and the ETH Zurich reported their findings in the Journal of the American Chemical Society, published online on 27 June 2023.

3D printed model of a carbon nanotube, the main building block for the new biosensors. Unlike in this 3D printed model, the real nanotubes are 100,000 times thinner than a human hair.
Straightforward customisation of carbon nanotube biosensors
The team used tubular nanosensors that were made of carbon and had a diameter of less than one nanometre. When irradiated with visible light, carbon nanotubes emit light in the near-infrared range. Near-infrared light is not visible to the human eye. However, it is perfect for optical applications, because the level of other signals in this range is highly reduced. In earlier studies, Sebastian Kruss’ team had already shown how the fluorescence of nanotubes can be manipulated in order to detect vital biomolecules. Now, the researchers searched for a way to customise the carbon sensors for use with different target molecules in a straightforward manner.
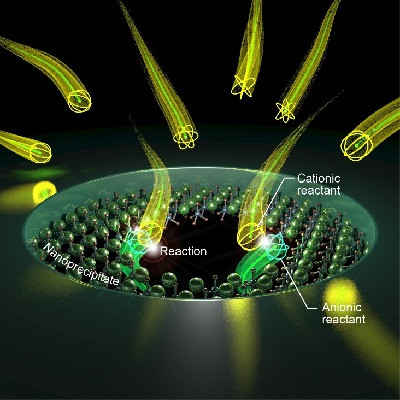
The key to success were DNA structures with so-called guanine quantum defects. This involved linking DNA bases to the nanotube to create a defect in the crystal structure of the nanotube. As a result, the fluorescence of the nanotubes changed at the quantum level. Additionally, the defect acted as a molecular handle that allowed to introduce a detection unit, which can be adapted to the respective target molecule for the purpose of identifying a specific viral or bacterial protein. “Through the attachment of the detection unit to the DNA anchors, the assembly of such a sensor resembles a system of building blocks – except that the individual parts are 100,000 times smaller than a human hair,” outlines Sebastian Kruss.

Experimental setup for the production of the guanine defects: LEDs and the photosensitizer rose bengal are used to produce a reactive form of oxygen that can selectively link certain DNA bases to the nanotube.
Sensor identifies different bacterial and viral targets
The group showcased the new sensor concept using the SARS CoV-2 spike protein as an example. To this end, the researchers used aptamers, that bind to the SARS CoV-2 spike protein. “Aptamers are folded DNA or RNA strands. Due to their structure, they can selectively bind to proteins,” explains Justus Metternich. “In the next step, one could transfer the concept to antibodies or other detection units.”
The fluorescent sensors indicated the presence of the SARS-CoV-2 protein with a high degree of reliability. The selectivity of sensors with guanine quantum defects was higher than the selectivity of sensors without such defects. Moreover, the sensors with guanine quantum defects were more stable in solution. “This is an advantage if you think about measurements beyond simple aqueous solutions. For diagnostic applications, we have to measure in complex environments e.g. with cells, in the blood or in the organism itself,” says Sebastian Kruss, who heads the Functional Interfaces and Biosystems Group at Ruhr University Bochum and is a member of the Ruhr Explores Solvation Cluster of Excellence (RESOLV) and the International Graduate School of Neuroscience.
Read the original article on Ruhr University Bochum.