

| Date | 25th, Sep 2023 |
|---|
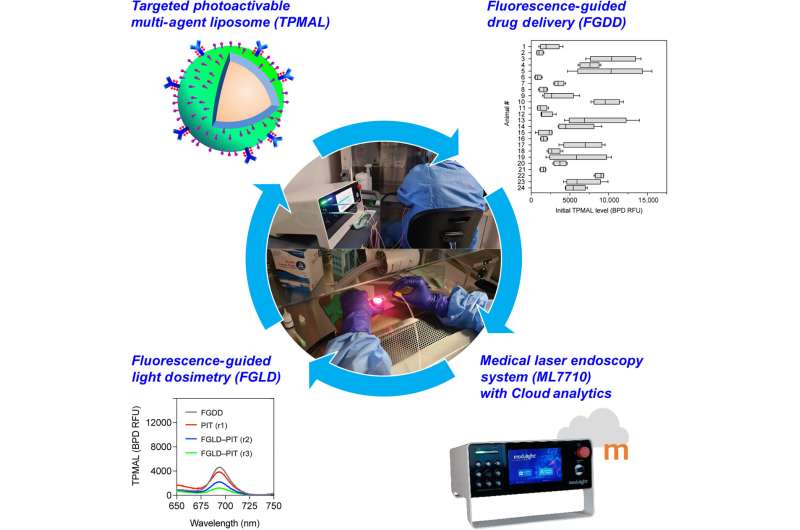 Workflow schematic. This shows the overall workflow of integrating targeted nanomedicine, medical laser endoscopy system, and fluorescence-guided intervention to improve the safety, efficacy, and consistency of photoimmunotherapy (PIT) for peritoneal metastasis. The targeted photo-activable multi-agent nanoliposome (TPMAL) co-delivers fluorophores, photosensitizer immunoconjugates (PICs), and chemotherapy drugs at high payloads for combined imaging, PIT, and chemotherapy of peritoneal tumors. A medical laser endoscopy system (ML7710) coupled with Modulight Cloud was engineered to perform PIT and fluorescence-guided intervention in the abdominal cavity of the patient. Specifically, fluorescence-guided drug delivery (FGDD) can reveal interpatient variability in PIC uptake, which prompts the need for intraoperative fluorescence-guided light dosimetry (FGLD) adjustment in study subjects. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi3441
Workflow schematic. This shows the overall workflow of integrating targeted nanomedicine, medical laser endoscopy system, and fluorescence-guided intervention to improve the safety, efficacy, and consistency of photoimmunotherapy (PIT) for peritoneal metastasis. The targeted photo-activable multi-agent nanoliposome (TPMAL) co-delivers fluorophores, photosensitizer immunoconjugates (PICs), and chemotherapy drugs at high payloads for combined imaging, PIT, and chemotherapy of peritoneal tumors. A medical laser endoscopy system (ML7710) coupled with Modulight Cloud was engineered to perform PIT and fluorescence-guided intervention in the abdominal cavity of the patient. Specifically, fluorescence-guided drug delivery (FGDD) can reveal interpatient variability in PIC uptake, which prompts the need for intraoperative fluorescence-guided light dosimetry (FGLD) adjustment in study subjects. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi3441
Fluorescence-guided intervention strategies can improve standard therapies to detect and treat microscopic tumors to thereby prevent lethal recurrence. Cancer biologists have made tremendous progress in photoimmunotherapy and nanotechnology to treat metastasis, although the effects of such techniques are limited by heterogeneous effects.
In a new report published in Science Advances, Barry J. Liang, and a team of researchers in bioengineering, cell biology and photomedicine at the University of Maryland, Baltimore, Harvard Medical School U.S. and the Modulight Corporation, Finland, integrated three technical advances for fluorescence-guided intervention in targeted photo-activatable multiagent liposome laser endoscopy for improved photoimmunotherapy.
The photoactivatable multiagent liposome contained a nanoliposome labeled with fluorophores to track and photosensitize immunoconjugates for photoimmunotherapy. The researchers conducted fluorescence-guided drug delivery during the experiments and fluorescence-guided light dosimetry to investigate peritoneal carcinomatosis in mouse models.
Fluorescence-guided drug delivery methods revealed that the targeted photoactivatable multiagent liposome enhanced drug delivery to metastases increased by 14-fold. The team combined the interventional methods to vary the treatment response for tumor control without side-effects.
Peritoneal metastasis or incomplete resection and drug resistance can make advanced ovarian cancer virtually incurable with the existing approaches in surgery and chemotherapy. While tumor recurrence is nearly universal, the five-year survival rate of 30% has not significantly changed in the past three decades.
At diagnosis, up to 70% of these patients are in advanced stages. The primary mechanism of serous carcinoma metastasis involves the high-grade deposition of numerous cancer nodules throughout the abdominal cavity. Women with advanced ovarian cancer who undergo surgery and chemotherapy have achieved complete remission, although the patients relapse due to residual sub-mm lesions.
While such aggregates are difficult to detect, they can develop resistance to standard treatments, therefore radical approaches that combine targeted therapy, imaging and monitoring should address drug-resistant micro-metastases.
Although intraoperative photodynamic therapy for peritoneal carcinomatosis using non-targeted photosensitizers and a fixed light dose combination is safe for clinical use, the technology has not yet achieved complete responses or long-term tumor control due to tumor heterogeneity and a lack of specificity during the uptake of photosensitizer.
In this work, Liang and colleagues used targeted photoactivatable multiagent liposomes (TPMAL) for photochemotherapy engineered with molecular targeting and fluorescence-tracking features. The scientists integrated a laser endoscopy system to advance a two-pronged approach to ensure TPMAL-assisted photodynamic therapy for safe, and customized drug delivery. The outcomes highlight the efficiency and safety of photoimmunotherapy to reduce the metastatic burden in vivo.
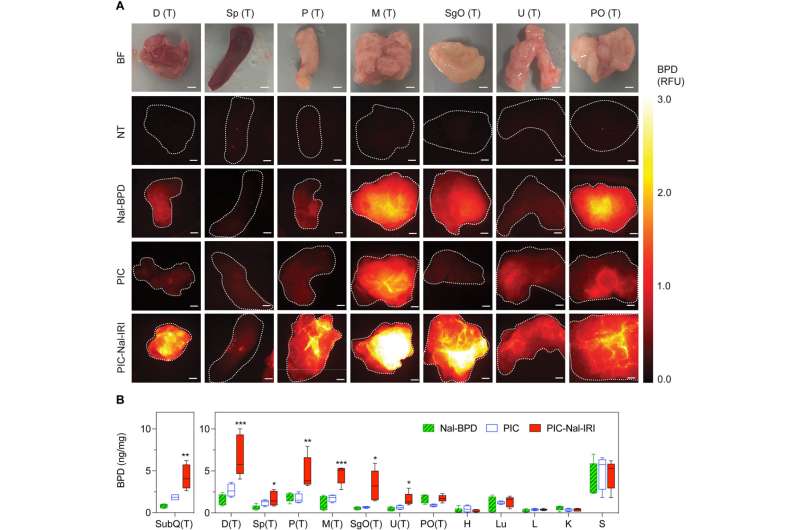 Biodistribution of TPMAL (PIC-Nal-IRI), PIC, and Nal-BPD in peritoneal metastases and subcutaneous tumors. At 14 days after OVCAR-5 implantation, mice were intraperitoneally injected with PIC-Nal-IRI, PIC, or Nal-BPD (BPD: 0.25 mg/kg). Tissues were excised and imaged for BPD fluorescence according to Materials and Methods at 24 hours after injection. (A) Representative bright-field (BF) and fluorescence images of tissues with peritoneal metastases from no treatment (NT), Nal-BPD, PIC, and PIC-Nal-IRI groups. Scale bar, 1.5 mm. (B) Quantification of BPD accumulation in excised tissues at 24 hours after injection with BPD standards of known concentrations. Asterisks denote significance compared to PIC group. Data are represented as mean ± SEM values (N ≥ 3 animals per group, *P < 0.05, **P < 0.01, ***P
Biodistribution of TPMAL (PIC-Nal-IRI), PIC, and Nal-BPD in peritoneal metastases and subcutaneous tumors. At 14 days after OVCAR-5 implantation, mice were intraperitoneally injected with PIC-Nal-IRI, PIC, or Nal-BPD (BPD: 0.25 mg/kg). Tissues were excised and imaged for BPD fluorescence according to Materials and Methods at 24 hours after injection. (A) Representative bright-field (BF) and fluorescence images of tissues with peritoneal metastases from no treatment (NT), Nal-BPD, PIC, and PIC-Nal-IRI groups. Scale bar, 1.5 mm. (B) Quantification of BPD accumulation in excised tissues at 24 hours after injection with BPD standards of known concentrations. Asterisks denote significance compared to PIC group. Data are represented as mean ± SEM values (N ≥ 3 animals per group, *P < 0.05, **P < 0.01, ***P 