Soon, a mouthwash of iron oxide nanoparticles could save people a trip to the dentist. A new research study in rats shows that such a mouthwash can thwart and stop cavities by disrupting destructive bacterial biofilms that grow on teeth (Nat. Comm. 2018, DOI: 10.1038/s41467-018-05342).
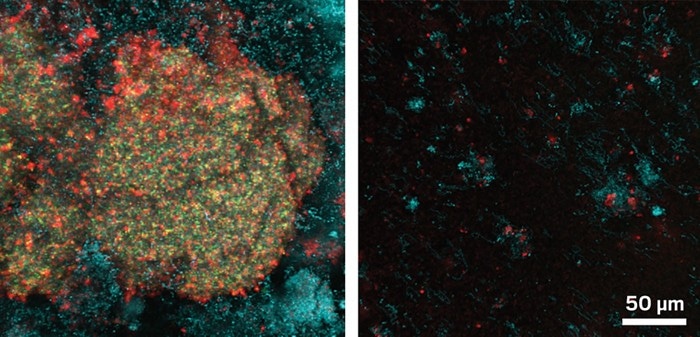 Biofilms (indicated by red and green) grown on rat tooth enamel (left) were broken up by a combination of iron oxide nanoparticles and hydrogen peroxide (right). (Image credit: Nat. Comm.)
Biofilms (indicated by red and green) grown on rat tooth enamel (left) were broken up by a combination of iron oxide nanoparticles and hydrogen peroxide (right). (Image credit: Nat. Comm.)
The scientists, guided by Hyun (Michel) Koo from the University of Pennsylvania School of Dental Medicine, used a formulation called ferumoxytol, which contains iron oxide nanoparticles coated with carboxymethyl dextran. Fermuoxytol is permitted by the U.S. Food & Drug Administration for treating iron deficiency.
The team planned to test the teeth-protecting abilities of these iron oxide nanoparticles because they can break down hydrogen peroxide to develop hydroxyl radicals that can disturb biofilms. Koo and colleagues developed a test mouthwash therapy involving solutions of ferumoxytol and hydrogen peroxide. They used nanoparticle doses that were below 1% of what is used to treat iron-deficiency, Koo says.
Over a period of three weeks, the scientists used both solutions twice a day to rinse the mouths of rats with cavities that mimic extreme childhood dental decay. In contrast to animals that did not get the mouthwash, treated rats saw substantial improvements in their dental decay. The mouthwash both stopped the progression of current damage and stopped damage to smooth, healthy teeth.
The method holds promise, says Zainab Hosseini-Doust, a bioengineer at McMaster University. “In most cases, the only effective way of eradicating a biofilm is physical removal of the infected biomaterial,” she says. “What’s interesting about this work is the ability of the developed nanoparticles to degrade the biofilm matrix from within.”
Hosseini-Doust is keen to know more about how the nanoparticles interact with healthy oral biofilms. “The health of our teeth and oral cavity is highly dependent on the community of good bacteria living in our mouth. Any nonspecific antibacterial treatment will inevitably affect our good bacteria,” she says.
The team is currently involved in developing formulations with higher catalytic activity, and ultimately aims to test the effectiveness of the mouthwash in humans, Koo says.
Source: https://cen.acs.org

