

| Date | 4th, Sep 2018 |
|---|
 Experts in the Nanoscale and Microscale Research Centre at the University of Nottingham have taken a first peak into the private life of atomic clusters. Credit: University of Nottingham
Experts in the Nanoscale and Microscale Research Centre at the University of Nottingham have taken a first peak into the private life of atomic clusters. Credit: University of Nottingham
Experts in the Nanoscale and Microscale Research Centre (nmRC) at the University of Nottingham have taken a first peak into the private life of atomic clusters.
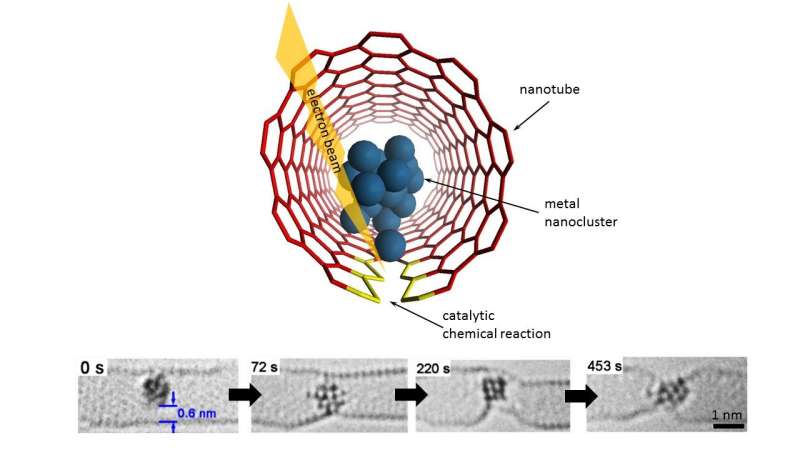
Having already succeeded in 'filming' inter-molecular chemical reactions—using the electron beam of a transmission electron microscope (TEM) as a stop-frame imaging tool they have now achieved time-resolved imaging of atomic-scale dynamics and chemical transformations promoted by metal nanoclusters. This has enabled them to rank 14 different metals both in order of their bonding with carbon and their catalytic activity, showing significant variation across the Periodic Table of Elements.
Their latest work, 'Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts', has been published in Nature Communications. Andrei Khlobystov, Professor of Nanomaterials and Director of nmRC, said: "Thanks to the recent advances in microscopy and spectroscopy we now know a great deal about the behaviour of molecules and atoms. However, the structure and dynamics of atomic-scale clusters of metallic elements remains a mystery. The complex atomic dynamics revealed directly by imaging in real time sheds light on atomistic workings of nanocatalysts."
Contribution to global GDP
The atomic-scale dynamics of metal nanoclusters determine their functional and chemical properties such as catalytic activity—their ability to increase the rate of a chemical reaction. Many key industrial processes currently rely on nanocatalysts such as water purification; fuel cell technologies; energy storage; and bio-diesel production.
Professor Khlobystov said: "With catalytic chemical reactions contributing substantially to the global GDP, understanding the dynamic behaviour of nanoclusters at the atomic level is an important and urgent task. However, the combined challenge of non-uniform structures of nanocatalysts—for example, distribution of sizes, shapes, crystal phases—coexisting within the same material and their highly dynamic nature—nanoclusters undergo extensive structural and, in some cases, chemical transformations during catalysis—makes elucidation of the atomistic mechanisms of their behaviour virtually impossible."
From single-molecule dynamics to atomic clusters
Professor Khlobystov led the Anglo-German collaboration that harnessed the impact of the electron beam (e-beam) in the transmission electron microscopy (TEM) for imaging single-molecule dynamics. By employing the e-beam simultaneously as an imaging tool and a source of energy to drive chemical reactions they succeeded at filming reactions of molecules. The research was published last year in ACS Nano, a flagship nanoscience and nanotechnology journal, and selected as ACS Editor's Choice due to its potential for broad public interest.
