Sep 05, 2018
(Nanowerk News) Gold is the noblest metal—the most resistant to oxidation. However, nano-size gold has a unique ability to perform as a catalyst, even at low temperatures. The underlying mechanism for this size-dependent change in properties has puzzled scientists since the phenomenon was discovered in the late 1980s.
A team of researchers, including Yingge Du, Chongmin Wang, and Jun Li of Pacific Northwest National Laboratory, set out to address this question using state-of-the-art aberration-corrected environmental transmission electron microscopy.
Their work has revealed new insights about the exceptional catalytic properties of ultrasmall gold particles when they are exposed to reactant gas.
Details of their findings have been published in a Proceedings of the National Academy of Sciences paper ("Size-dependent dynamic structures of supported gold nanoparticles in CO oxidation reaction condition").
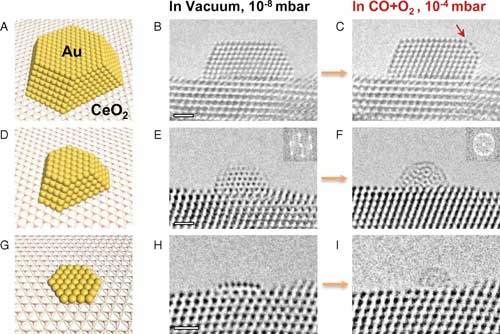 Dynamic structural changes of various gold nanostructures supported on CeO2(111) upon exposure to the reactant gases. (A–C) Model (A) and HRTEM images (B and C) of a 4-nm Au NP with ∼1,000 atoms; the arrow in C indicates the Au(100) surface reconstruction. (D–F) The
Dynamic structural changes of various gold nanostructures supported on CeO2(111) upon exposure to the reactant gases. (A–C) Model (A) and HRTEM images (B and C) of a 4-nm Au NP with ∼1,000 atoms; the arrow in C indicates the Au(100) surface reconstruction. (D–F) The
 Dynamic structural changes of various gold nanostructures supported on CeO2(111) upon exposure to the reactant gases. (A–C) Model (A) and HRTEM images (B and C) of a 4-nm Au NP with ∼1,000 atoms; the arrow in C indicates the Au(100) surface reconstruction. (D–F) The
Dynamic structural changes of various gold nanostructures supported on CeO2(111) upon exposure to the reactant gases. (A–C) Model (A) and HRTEM images (B and C) of a 4-nm Au NP with ∼1,000 atoms; the arrow in C indicates the Au(100) surface reconstruction. (D–F) The 
