

| Date | 12th, Nov 2018 |
|---|
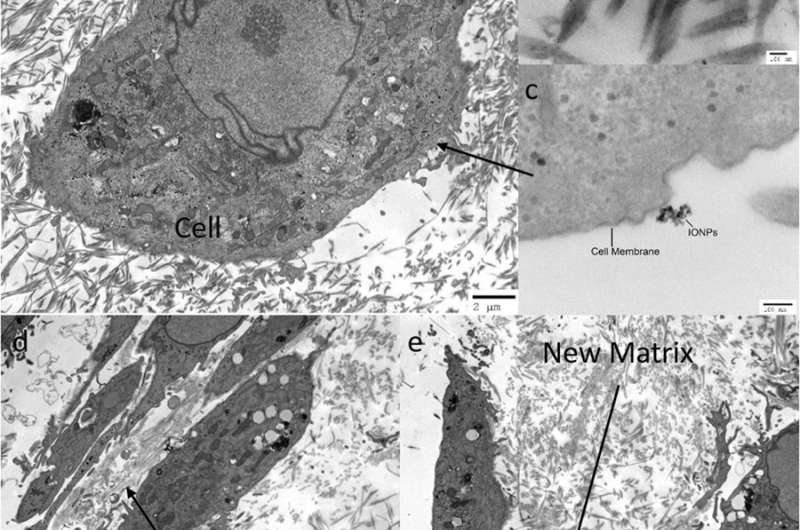 a) Microstructure of cell loaded collagen scaffolds examined under TEM. Cells are surrounded by the collagen matrix. The collagen fibrils showed no preferred direction around the cells under control conditions, b) IONPs can be identified in the collagen matrix and c) close to the cell membranes. After 14 days a new matrix was synthesized by the cells, d) without SMFs and e) with SMFs. Both conditions led to new matrix synthesis after 14 days, without a significant difference. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
a) Microstructure of cell loaded collagen scaffolds examined under TEM. Cells are surrounded by the collagen matrix. The collagen fibrils showed no preferred direction around the cells under control conditions, b) IONPs can be identified in the collagen matrix and c) close to the cell membranes. After 14 days a new matrix was synthesized by the cells, d) without SMFs and e) with SMFs. Both conditions led to new matrix synthesis after 14 days, without a significant difference. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
The cellular and molecular mechanisms of magnetic stimulation-based bone regeneration require further understanding at present. To evaluate the phenomenon in the lab, a three-dimensional (3-D) native collagen model was recently developed via plastic compression to produce a cellular, dense and mechanically strong collagen structure. To produce cell-laden models in the study, Zhiyu Yuan and colleagues incorporated osteoblast cells (MG-63 cell line) and magnetic iron oxide nanoparticles (IONPs) into the collagen gels. Using 3-D printing, a magnetic bioreactor was designed and fabricated to support cell growth under static magnetic fields (SMFs). Using polymerase chain reaction (PCR), the researchers determined the impact of SMFs on the regulation and expression of genes related to osteogenesis including runt-related transcription factor 2 (Runx2), osteonectin (ON) and bone morphogenetic proteins 2 and 4 (BMP-2 and BMP-4).
Now published in Scientific Reports, the results demonstrated that SMFs, IONPs and the collagen matrix were able to stimulate the proliferation, alkaline phosphatase production and mineralization of osteoblasts. The process was enabled by influencing matrix-cell interactions to influence the expression of Runx2, ON, BMP-2 and BMP-4. The collagen model offered insight to progressively form a novel mineralized 3-D bone model and understand magnetic stimulation on osteogenesis. Additional studies can be conducted with the model for applications in tissue engineering and regenerative medicine.
In the U.K. alone, the number of patients suffering from bone fracture have a substantial economic impact on the quality of life as evidenced with costs to the National Health Services (NHS). During bone injury, the biological and mechanical process of physiological regeneration replaces the injured bone with new bone at the site of injury. The metabolic process is complicated and requires the interaction of many factors, including growth and differentiation factors such as hormones, cytokines and extracellular components; meanwhile inadequate or interrupted factors can lead to delayed healing or impaired/non-union of bone.
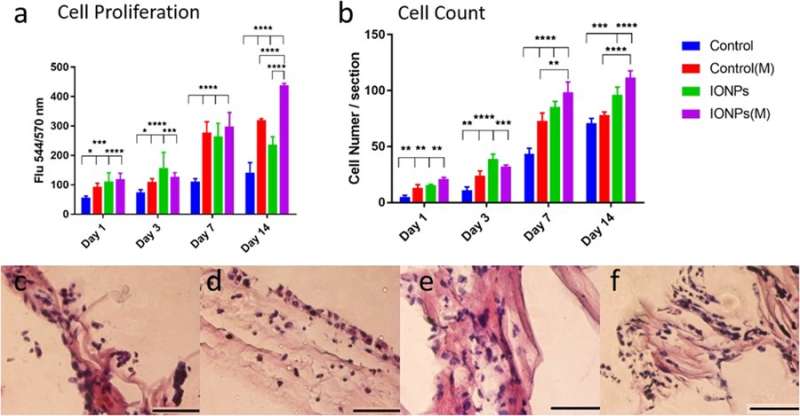 a) comparison of MG-63 cell proliferation when cultured with/without the incorporation of IONPs with exposure of SMFs (M) and without the exposure of SMFs. Cell proliferation can be enhanced with SMFs by incorporating IONPs, indicating a stimulating effect, b) Comparing the cell numbers of the MG-63 cell lines from histology images with or without SMFs. The cellular responses of the MG-63 cell line cultured on PC collagen scaffold were also examined via histology for conditions c) without SMFs and without IONPs, d) with SMFs, e) with IONPs and e) combining SMFs and IONPs. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
a) comparison of MG-63 cell proliferation when cultured with/without the incorporation of IONPs with exposure of SMFs (M) and without the exposure of SMFs. Cell proliferation can be enhanced with SMFs by incorporating IONPs, indicating a stimulating effect, b) Comparing the cell numbers of the MG-63 cell lines from histology images with or without SMFs. The cellular responses of the MG-63 cell line cultured on PC collagen scaffold were also examined via histology for conditions c) without SMFs and without IONPs, d) with SMFs, e) with IONPs and e) combining SMFs and IONPs. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
In the study, the authors used a novel, multifunctional biomimetic 3-D collagen model for use as an in vitro platform to study mechanisms of magnetic stimulation on osteogenesis. To produce a range of cell-laden models, the researchers introduced internal stimuli (iron oxide nanoparticles) and external (static magnetic fields, SMFs) stimuli in to the system. The biomimetic material was engineered via the fabrication of nano- and microstructures using plastic compression, according to a previously established protocol. In order to evaluate biological behaviors of osteoblasts, including their proliferation, differentiation, mineralization, gene expression and microstructure analysis, the scientists cultured the collagen model in a magnetic bioreactor for up to 42 days.
In the study, cell proliferation of the osteoblast cell line MG-63 was assessed using the alamarBlue assay. At day 14, the researchers observed a significant difference between SMFs alone, IONPs alone and in their combination on cell proliferation. Histology techniques were employed to examine cellular responses within the collagen scaffolds and understand the role of SMFs and IONPs on cell proliferation. Visualization was followed by quantitative analysis of cell numbers, results indicated that incorporation of IONPs prolonged the effect of SMFs.
Similarly, cell differentiation was observed with alkaline phosphatase (ALP) activities for collagen scaffolds, with or without the incorporation of IONPs. As before, when the effect of IONPs and SMFs were combined the ALP production was significantly stimulated compared to treatment with SMFs alone and IONPs alone. Cell mineralization was also observed thereafter and quantified in the cell-seeded collagen scaffold. After 42 days all samples were stained with ARS staining to indicate complete mineralization. In contrast, combining SMFs and IONPs were unable to promote mineralization.
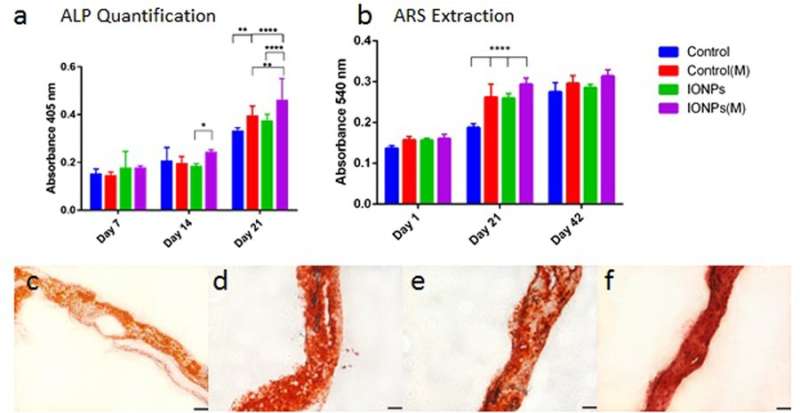 a) comparing ALP production of MG-63 cell line when cultured within collagen scaffolds with or without the incorporation of IONPs, with exposure to SMFs (M) and without exposure to SMFs. b) Comparing cell mineralization by extracting and quantifying ARS staining from scaffolds treated with or without SMFs exposure. Cell-loaded collagen scaffolds were integrated with IONPs (100 µg/ml) results were collected across 1, 21 and 42 days of culture. c) ARS staining of collagen scaffold in the absence of SMFs and IONPs, d) with SMFs, e) with IONPs, and f) with SMFs and IONPs. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
a) comparing ALP production of MG-63 cell line when cultured within collagen scaffolds with or without the incorporation of IONPs, with exposure to SMFs (M) and without exposure to SMFs. b) Comparing cell mineralization by extracting and quantifying ARS staining from scaffolds treated with or without SMFs exposure. Cell-loaded collagen scaffolds were integrated with IONPs (100 µg/ml) results were collected across 1, 21 and 42 days of culture. c) ARS staining of collagen scaffold in the absence of SMFs and IONPs, d) with SMFs, e) with IONPs, and f) with SMFs and IONPs. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
Thereafter, the scientists conducted studies to understand responses of cell-seeded collagen scaffolds to SMFs and IONPs at the molecular level. For this, the expression levels of Runx2, ON, BMP-2 and BMP-4 were quantified using real-time quantitative polymerase chain reaction (RT-qPCR). A 7-day treatment of SMFs alone did not have an effect on the expression of Runx2, whereas increased expression was found when SMFs were combined with IONPs, demonstrating the collagen matrix mediated Runx2 expression during osteogenesis. At day 7 the level of ON expression in the samples treated with IONPs, SMFs and both were higher than in the control within a short period of time. During the expression of BMP-2 and BMP-4, similar results were observed across 7-14 days.
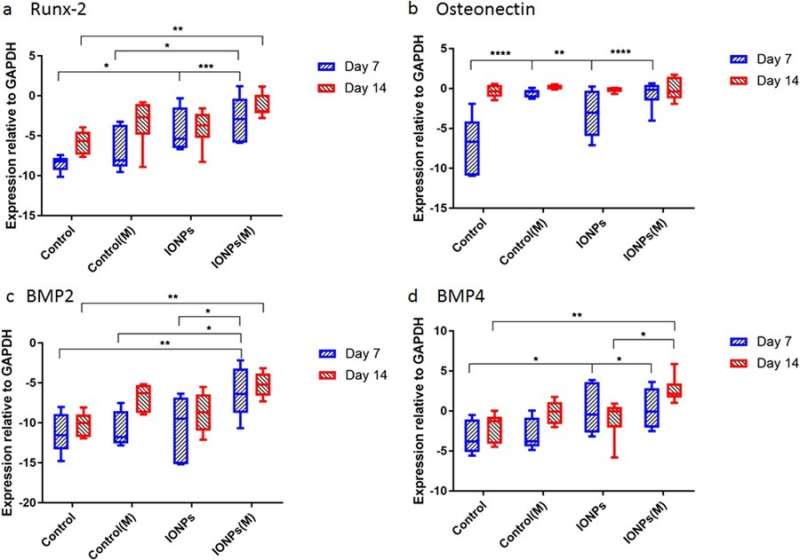 Gene expression for a) Runx2, b) osteonectin (ON), c) BMP-2 and d) BMP-4 normalized with the control GAPDH after 7 and 14 days of cell culture. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
Gene expression for a) Runx2, b) osteonectin (ON), c) BMP-2 and d) BMP-4 normalized with the control GAPDH after 7 and 14 days of cell culture. Credit: Scientific Reports, doi: 10.1038/s41598-018-33455-2.
The study mainly demonstrated osteogenesis as a complex process mediated by successive activation and expression of several key genes, including Runx2, ON, BMP-2 and BMP-4. Typically, Runx2 upregulates the expression of protein genes related to the bone matrix to promote bone formation in vitro and in vivo. Accordingly, the study showed that SMF exposure may induce the cell line to proliferate by modulating early expression of Runx2, to accelerate osteogenesis. When IONPs were incorporated under SMFs, the expression of osteonectin (ON) gene increased, linking collagen phases with bone mineral to initiate normal skeletal tissue mineralization. Similarly, both BMP-2 and BMP-4 were only increased in the study during combined cell culture with SMFs and IONPs.
In this way, the authors developed and tested a biomimetic 3-D collagen model that could progressively mineralize to subsequently form a novel bone model in vitro. The model was incorporated with osteoblast cells and nanoparticles with the ability to respond to external magnetic stimulations. The biomimetic collagen model was engineered via plastic compression to integrate the MG-63 cell line and demonstrate reproducible and consistent results combined with SMFs and IONPs. The cell-matrix interactions successfully upregulated the expression of key genes related to osteogenesis. The authors intend to develop the 3-D model further to serve as a superior platform to investigate biological behaviors in vitro with potential applications in tissue engineering and regenerative medicine.
More information: Zhiyu Yuan et al. Development of a 3D Collagen Model for the In Vitro Evaluation of Magnetic-assisted Osteogenesis, Scientific Reports (2018). DOI: 10.1038/s41598-018-33455-2 Jon Dobson.
Remote control of cellular behaviour with magnetic nanoparticles, Nature Nanotechnology (2008). DOI: 10.1038/nnano.2008.39
Aurelie G. Andrianarivo et al. Growth on type I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells, Journal of Cellular Physiology (2005). DOI: 10.1002/jcp.1041530205
© 2018 Science X Network
Citation: Developing a 3-D collagen model to test magnetic-assisted osteogenesis in vitro (2018, November 12) retrieved 22 August 2022 from https://phys.org/news/2018-11-d-collagen-magnetic-assisted-osteogenesis-vitro.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
