Feb 28, 2019
(Nanowerk News) Nanostructures based on carbon are promising materials for nanoelectronics. However, to be suitable, they would often need to be formed on non-metallic surfaces, which has been a challenge – up to now. Researchers at Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) have found a method of forming nanographenes on metal oxide surfaces.
Their research, conducted within the framework of collaborative research centre 953 – Synthetic Carbon Allotropes funded by the German Research Foundation (DFG), has now been published in the journal Science ("Fluorine-programmed nanozipping to tailored nanographenes on rutile TiO2 surfaces").
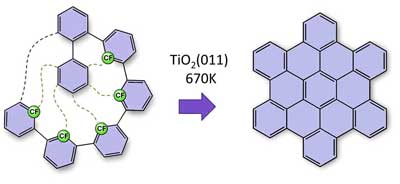 The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed. (Image: FAU/Konstantin Amsharov)
Two-dimensional, flexible, tear-resistant, lightweight, and versatile are all properties that apply to graphene, which is often described as a miracle material. In addition, this carbon-based nanostructure has unique electrical properties that make it attractive for nanoelectronic applications.
Depending on its size and shape, nanographene can be conductive or semi-conductive – properties that are essential for use in nanotransistors. Thanks to its good electrical and thermal conductivity, it could also replace copper (which is conductive) and silicon (which is semi-conductive) in future nanoprocessors.
The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed. (Image: FAU/Konstantin Amsharov)
Two-dimensional, flexible, tear-resistant, lightweight, and versatile are all properties that apply to graphene, which is often described as a miracle material. In addition, this carbon-based nanostructure has unique electrical properties that make it attractive for nanoelectronic applications.
Depending on its size and shape, nanographene can be conductive or semi-conductive – properties that are essential for use in nanotransistors. Thanks to its good electrical and thermal conductivity, it could also replace copper (which is conductive) and silicon (which is semi-conductive) in future nanoprocessors.
 The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed. (Image: FAU/Konstantin Amsharov)
Two-dimensional, flexible, tear-resistant, lightweight, and versatile are all properties that apply to graphene, which is often described as a miracle material. In addition, this carbon-based nanostructure has unique electrical properties that make it attractive for nanoelectronic applications.
Depending on its size and shape, nanographene can be conductive or semi-conductive – properties that are essential for use in nanotransistors. Thanks to its good electrical and thermal conductivity, it could also replace copper (which is conductive) and silicon (which is semi-conductive) in future nanoprocessors.
The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed. (Image: FAU/Konstantin Amsharov)
Two-dimensional, flexible, tear-resistant, lightweight, and versatile are all properties that apply to graphene, which is often described as a miracle material. In addition, this carbon-based nanostructure has unique electrical properties that make it attractive for nanoelectronic applications.
Depending on its size and shape, nanographene can be conductive or semi-conductive – properties that are essential for use in nanotransistors. Thanks to its good electrical and thermal conductivity, it could also replace copper (which is conductive) and silicon (which is semi-conductive) in future nanoprocessors.

