A very small structure from 32 gold atoms has been successfully synthesized by scientists. Precisely, 12 gold atoms enclosed by a shell of 20 extra gold atoms form the core of this unique nanocluster.
 (Image credit: Wiley)
(Image credit: Wiley)
Reporting their study in the Angewandte Chemie journal, the researchers described that the extraordinary stability of this nanocluster occurs because of electronic interactions with phosphine and amido ligands adhered to its surface.
Clusters are aggregates of several metal atoms. They are increasingly utilized in nanotechnology, bioscience, and catalysis. “Golden cages” have turned out to be a novel class of clusters with extraordinary structural properties ever since the discovery of a 20 gold atom-pyramidal structure. A cluster of 32 gold atoms was predicted to be extremely stable and would look like C60 fullerene in terms of structure. C60 fullerene is a hollow sphere containing 60 carbon atoms organized into a 5- and 6-membered ring structure, similar to the facets of a regular soccer ball. It has been predicted that “golden fullerenes” will have a wide spectrum of promising applications, including catalysts, molecular labels, and transporters.
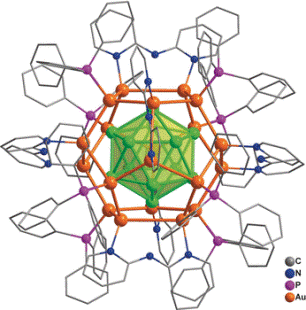
For the first time, a research team, headed by Jun Li and Quan-Ming Wang (Tsinghua University, Beijing, China) has successfully synthesized a unique nanocluster composed of 32 gold atoms in solution. In this process of synthesis, gold-containing precursors were directly reduced, [Au32(Ph3P)8(dpa)6]+[SbF6−]2 is the formula of the compound where Ph3P = triphenylphosphine ligand and dpa = 2,2’-bipyridylamido ligand. Within the structure, 12 gold atoms are arranged in a cage of 20 gold atoms—defined as Au12@Au20—that is shielded by six dpa and eight Ph3P ligands adhered to the surface. Using a combination of computer calculations and analytical techniques, the researchers were able to fully establish the structure, such as its electronic structure, its chemical bonding relationships, and its geometry.
Within the novel cluster compound, the “golden core” is a hollow icosahedron (a shape having 20 triangular faces) composed of 12 gold atoms enclosed by a “shell” of 20 additional gold atoms in the form of a dodecahedron (a shape having 12 pentagonal faces). Moreover, the bonds existing between the core and the shell are extremely robust. All the eight Ph3P ligands adhered to shell’s eight gold atoms form a cube’s corners. The dpa ligands are organized in such a way that their midpoints are able to form the corners of an octahedron. Instead of being adhered through their amido groups, the dpa ligands are bound through a pair of nitrogen atoms in their two aromatic rings, each bridging two gold atoms.
“The geometric and electronic structures of the gold cluster depend very strongly on interactions with the ligands, as confirmed by our quantum chemical studies,” the scientists stated. “The dpa ligands in particular ensure the effective stabilization of the gold nanocluster. We anticipate that a rich variety of coinage-metal nanoclusters can be generated with the protection of amido ligands.”

