It is believed that hydrogen will serve as a clean energy for humans in the coming days. In this regard, electrochemical water splitting offers a potential process for producing hydrogen.
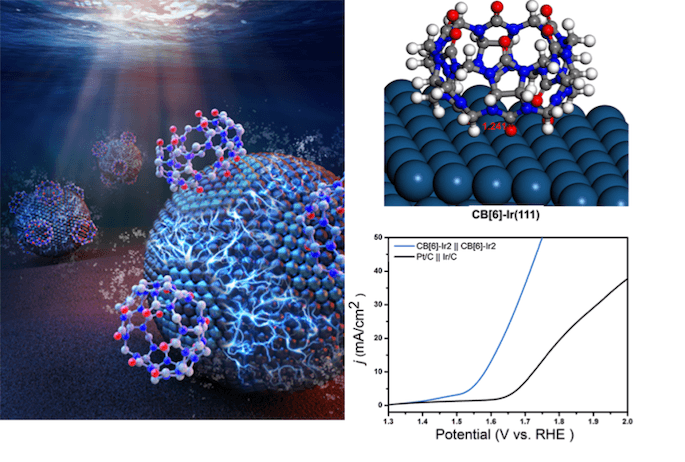 Schematic illustration of the CB[6]-Ir composite, the interaction between CB[6] and Ir and the superior water splitting performance as bifunctional electrocatalyst (Image credit: Prof. CAO’s group)
Schematic illustration of the CB[6]-Ir composite, the interaction between CB[6] and Ir and the superior water splitting performance as bifunctional electrocatalyst (Image credit: Prof. CAO’s group)
In acidic media, water splitting demands just a small overpotential for the evolution of cathodic hydrogen. The activity of low anodic oxygen evolution combined with serious deactivation of catalysts has restricted water splitting in acidic media.
In a recent study reported in ACS Energy Letters, a team of researchers headed by Professor CAO Rong from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences effectively produced a range of Iridium nanocrystals as extremely stable and efficient bifunctional electrocatalysts for water splitting process in acidic media.
Within the surface of the catalyst, the component of the active sites has been regulated using cucurbit[6]uril—an exclusive rigid macrocyclic compound—as both stabilizer and support.
The scientists observed that the improved CB[6]-Ir catalyst is one of the highest activities described in acidic media, achieving an overall water splitting current density of 10 mA/cm2 at just 1.56 V. The electrolytic cell also showed an excellent stability of at least 20 hours of continuous operation at 5 mA/cm2.
Spectroscopic outcomes provide a better understanding of the interaction between Iridium and cucurbit[6]uril and also the way the content variation of cucurbit[6]uril regulates the catalysts’ surface component.
With the help of density functional theory (DFT) calculation, the co-author and Professor ZHUANG Wei from FJIRSM, who deals in hypothetical computations on numerous catalytic systems, offered additional evidence to demonstrate the transfer of electrons between Iridium and cucurbit[6]uril. This is the first-ever report on the hypothetical computation of the cucurbit[n]uril-metal catalysts.
The latest study offers a novel approach to prepare efficient water splitting electrocatalysts and also demonstrates that cucurbit[n]uril have a vital role to play in the enhancement of electrocatalysts.
Source: http://english.cas.cn/

