

| Date | 29th, Jul 2019 |
|---|
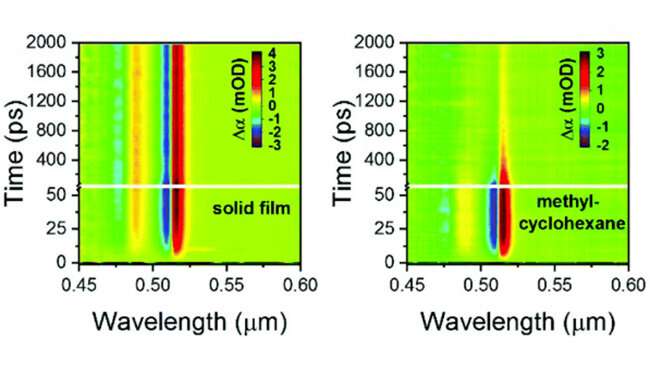 Two-dimensional maps of the temporal and spectral response of 4 ML CdSe NPLs to 3.46 μm pump excitation as a solid film (left) and in methylcyclohexane-d14. Credit: Argonne National Laboratory
Two-dimensional maps of the temporal and spectral response of 4 ML CdSe NPLs to 3.46 μm pump excitation as a solid film (left) and in methylcyclohexane-d14. Credit: Argonne National Laboratory
In a recent study published in Nanoscale, researchers show increases in cooling time for poorer hydrocarbon solvents compared to better solvents, indicate penetration of solvent into the ligand layer facilitates improved heat transfer to the matrix.
Heat transfer across hybrid organic-inorganic interfaces was measured by infrared pump, electronic probe spectroscopy (IPEP) which does not use electronic excitation, thereby removing any measurement artifacts.
Matching vibrational and molecular structure of ligands with surrounding solvents allows for quicker cooling of colloidal nanocrystals, which has implications for heat management in areas such as thermoelectrics.
Details
Femtosecond infrared pulses heat up organic ligands which transfer heat to attached inorganic nanoparticles, changing the bandgap on a 10 picosecond time-scale, followed by loss of heat to the surrounding solvent over hundreds of picoseconds.IPEP measurements were performed at CNM using ultrafast transient absorption spectroscopy.More information: Benjamin T. Diroll et al. Heating and cooling of ligand-coated colloidal nanocrystals in solid films and solvent matrices, Nanoscale (2019). DOI: 10.1039/C9NR01473J
Citation: Thermal management of hybrid nanoparticles (2019, July 29) retrieved 5 July 2022 from https://phys.org/news/2019-07-thermal-hybrid-nanoparticles.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
