

| Date | 19th, Aug 2019 |
|---|
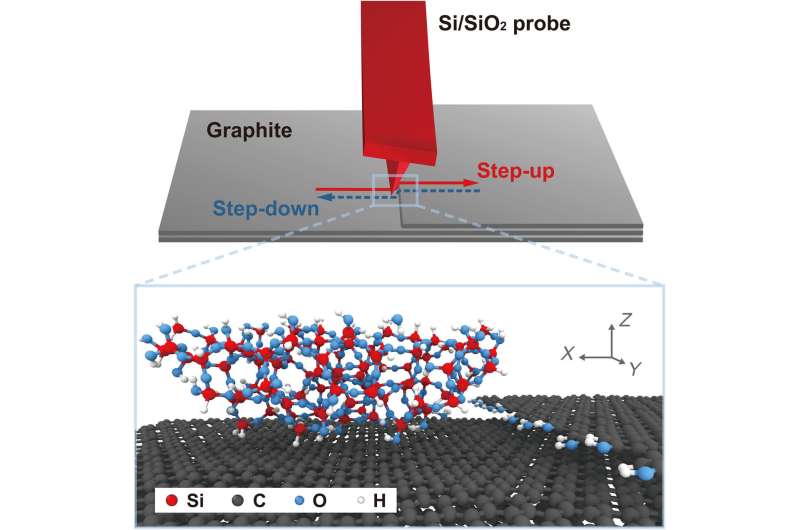 Schematic illustration and atomic-scale rendering of a silica AFM tip sliding up and down a single-layer graphene step edge on an atomically flat graphite surface. The silica tip model represents the native oxide at the apex of the Si AFM tip used in the experimental study. This model system enables both experimental and computational studies that isolate the chemical and physical origins of friction. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513
Schematic illustration and atomic-scale rendering of a silica AFM tip sliding up and down a single-layer graphene step edge on an atomically flat graphite surface. The silica tip model represents the native oxide at the apex of the Si AFM tip used in the experimental study. This model system enables both experimental and computational studies that isolate the chemical and physical origins of friction. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513
Friction results from a set of complex processes that act together to resist relative motion. Despite this complexity, friction is often described using simple phenomenological expressions that relate normal and lateral forces via the friction coefficient. The defined parameter encompasses multiple, sometimes competing effects. To better understand the origins of friction, Zhe Chen and an interdisciplinary team of researchers in the departments of chemical engineering, mechanical engineering and materials research studied a chemically and topographically well-defined interface between silica and graphite using a single-layer graphene step edge setup.
The research team identified the separate contributions of physical and chemical processes to friction and showed that a single friction coefficient could be separated into two terms corresponding to these effects. The results provided insight into the chemical and topographic origins of friction as an avenue of tuning surfaces by leveraging competing frictional processes. The findings are now published on Science Advances.
Friction occurs at the interface between any two solid surfaces in contact and moving at different speeds or directions. Since friction can correspond to wasted energy, scientists use the parameter to determine the efficiency and useful lifetime of all moving systems from biological to the aeronautical. Frictional force (Ff) is often linearly proportional to the applied load (L) at the microscale and the proportionality of this relationship, known as the coefficient of friction (COF) is symbolized by µ and expressed as Amonton's Law.
Adhesive forces (Fa) can become significant at the nanoscale to introduce an additional term for molecular mechanisms of tribology in thin films. While the expression is phenomenologically simple and has held value in experiments for decades, the actual mechanisms of determining the magnitude of the COF are very complicated. Physicists had previously proposed friction to have purely physical origins with related chemical processes to occur in sliding surfaces. But the interplay in the observed friction is thus far only poorly understood, since friction is typically associated with surface wear alone. In the present work, therefore, Chen et al. used a chemically and topographically well-defined interface to identify the contributions of physical and chemical processes to friction without accounting for surface wear to obtain fundamental insights into the origin of the frequently reported but poorly understood COF (coefficient of friction).
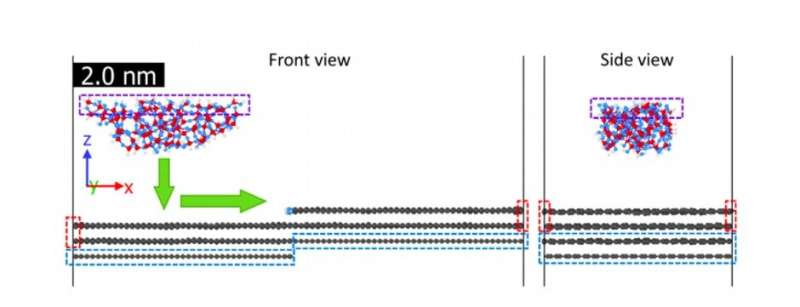 Front and side views of the MD simulation box. The box has periodic boundary conditions in the X and Y directions. The dashed boxes indicate regions in which atoms are treated as a rigid body (purple) or fixed in place (red and blue). The green arrows show the path of tip displacement during loading (downward movement) and sliding (lateral movement). Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
Front and side views of the MD simulation box. The box has periodic boundary conditions in the X and Y directions. The dashed boxes indicate regions in which atoms are treated as a rigid body (purple) or fixed in place (red and blue). The green arrows show the path of tip displacement during loading (downward movement) and sliding (lateral movement). Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
The scientists used a model system containing an atomic force microscopy (AFM) probe made of silicon referred to as a silica tip, and a graphite surface with a single-layer graphene step edge. The basal plane of graphite provided a chemically inert and defect-free flat surface. The exposed graphene sheet at the top was proportionate to the underlying layer, providing a topographically least corrugated surface for friction tests. The experimental system contained a single-layer graphene step edge on the graphite surface, to provide a well-defined topography with a height change of 0.34 nm across a distance corresponding to one chemical bond length to form an atomic step. The research team modelled the same system using reactive molecular dynamics (MD) simulations, recreating the apex of the silica tip on the topmost layers of graphene in the graphite, close to the step edge. They allowed for computational and experimental studies of the interfacial shear of a silica surface on an atomically flat surface, and on a chemically or topographically well-defined feature at the step, during the study. The experimental model agreed with the computational simulation to provide insight into the atomic-level origins of friction.
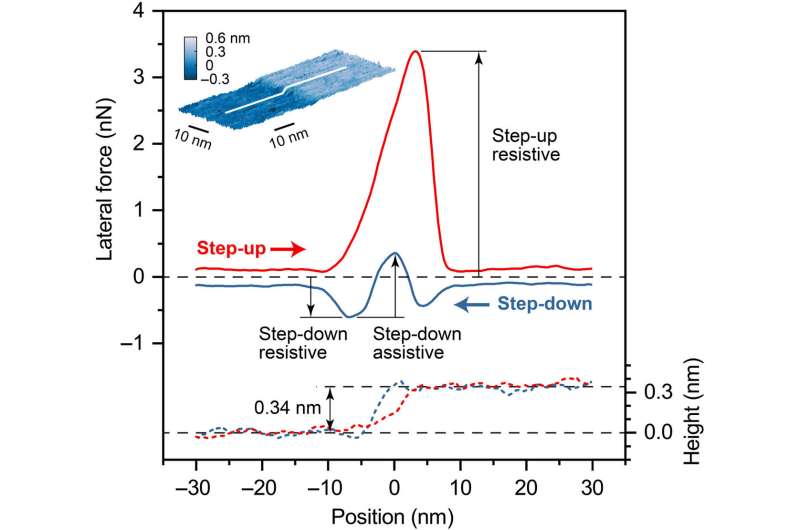 Lateral force (solid lines) and height profile (dashed lines) measured at the graphene step edge with a silica AFM tip. The normal force applied to the tip was 36.7 nN, and the sliding speed was 500 nm/s. In the step-up direction, the positive lateral force means that the graphene step edge is resisting tip sliding. In the step-down direction, the negative lateral force is resistive to the tip sliding and the positive (or upward deviation from the negative trend) force is assistive to the tip sliding. The inset is the AFM topographic image of the graphene step edge obtained after repeated friction measurements at applied normal forces varying from 7.3 to 36.7 nN (fig. S3A); the post-scan image shows no damage of the friction-tested region (white line). The height of the step edge is 0.34 nm, corresponding to the sum of the thickness of one graphene layer and the interlayer spacing between adjacent graphene layers. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
Lateral force (solid lines) and height profile (dashed lines) measured at the graphene step edge with a silica AFM tip. The normal force applied to the tip was 36.7 nN, and the sliding speed was 500 nm/s. In the step-up direction, the positive lateral force means that the graphene step edge is resisting tip sliding. In the step-down direction, the negative lateral force is resistive to the tip sliding and the positive (or upward deviation from the negative trend) force is assistive to the tip sliding. The inset is the AFM topographic image of the graphene step edge obtained after repeated friction measurements at applied normal forces varying from 7.3 to 36.7 nN (fig. S3A); the post-scan image shows no damage of the friction-tested region (white line). The height of the step edge is 0.34 nm, corresponding to the sum of the thickness of one graphene layer and the interlayer spacing between adjacent graphene layers. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
During measurements of the graphene step edge with a silica AFM tip, the research team obtained a COF of about 0.1, close to the value observed on various surfaces under elastic deformation tests. During step-down in the AFM tip based setup, Chen et al. observed more complicated friction responses in which the friction fluctuated during topographic height changes. The observed changes did not correspond to topography alone, but the team could not differentiate the chemical and physical effects in the system. To explore these origins, they analyzed friction as a function of load and observed load dependence of friction on the graphite terrace and at the graphene step edge from both experimental studies and simulations. The results confirmed that the simulations provided atomic insights into the interfacial processes of complex friction behaviors. They quantified the COF in the system with load bearing friction to isolate the chemical and physical contributions. The research team used the atomic-scale information observed in the simulations for additional insight.
To quantify physical contributions to friction in the reactive MD simulation, the scientists first used the shear strain of the silica tip. They then quantified the chemical contributions using the number of hydrogen bonds formed between the silica tip and graphite surface during the experiment. They did not observe significant physical or chemical interactions when the silica tip slid across the graphite basal plane, which they used to explain the experimental superlubricity of COF calculated (~0.003) in the study. However, during atomic step-up, the physical (strain) and chemical (hydrogen bonding) mechanisms synergistically enhanced resistance to sliding, causing the COF to become 100 folds greater at atomic step-up than at the basal plane of graphite. The scientists recorded similar observations for the step-down resistive force due to hydrogen bonding interactions.
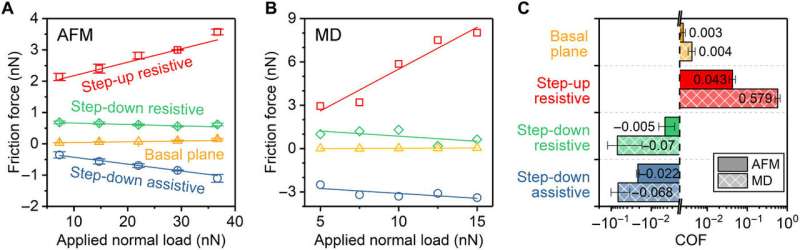 Load dependence of friction force and corresponding COF. (A) Friction force measured with the silica AFM tip under various applied normal loads. The step-up resistive, step-down resistive, and step-down assistive forces are determined. The mean and SD were calculated from values of multiple measurements, where each measurement involved averaging over 128 scans. The SDs of the experimental values are similar to or smaller than the size of symbols. (B) Friction force calculated from reactive MD simulations. Note that, for the step-down case, a positive assistive lateral force corresponds to a negative friction force. (C) COF calculated from the load dependence of friction force, which is the slope of the least squares fitting lines in (A) and (B). The error bar in (C) indicates the uncertainty in the calculated slope. Because friction force for the cases of step-down resistive and step-down assistive decreases as the applied load increases, negative COF is obtained. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
Load dependence of friction force and corresponding COF. (A) Friction force measured with the silica AFM tip under various applied normal loads. The step-up resistive, step-down resistive, and step-down assistive forces are determined. The mean and SD were calculated from values of multiple measurements, where each measurement involved averaging over 128 scans. The SDs of the experimental values are similar to or smaller than the size of symbols. (B) Friction force calculated from reactive MD simulations. Note that, for the step-down case, a positive assistive lateral force corresponds to a negative friction force. (C) COF calculated from the load dependence of friction force, which is the slope of the least squares fitting lines in (A) and (B). The error bar in (C) indicates the uncertainty in the calculated slope. Because friction force for the cases of step-down resistive and step-down assistive decreases as the applied load increases, negative COF is obtained. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
In this way, Zhe Chen and co-workers used COFs and MD simulations together, to provide insight into the physical and chemical origins of friction. They achieved superlubricity in the experimental setup when the strain induced by the topography and interlocking, as well as chemical bonding at the shear plane were negligible. The team observed large friction in the setup when the step-up above the 0.34 nm high graphene step edge caused combined physical effects from topography and chemical effects due to interfacial bonding. During step-down motion in the experiments, the negative topography change produced a force to assist sliding motion, while the chemical bonds between the oppositely moving surfaces produced a resistive force. The research team showed that balancing these two components could determine if the friction and the COF in an experimental system were ultimately positive or negative.
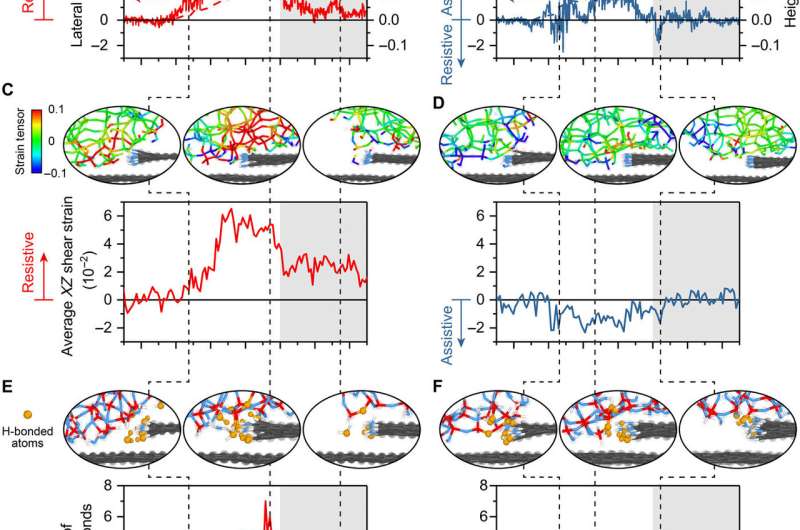 Reactive MD simulation showing the origins of chemical and physical effects on friction. (A and B) Lateral force, (C and D) shear strain of atoms in the silica where the sign indicates direction relative to sliding, and (E and F) number of hydrogen bonds formed between the graphene step edge and the silica, calculated from simulations as a function of center-of-mass position of the tip with respect to the graphene step edge for (A, C, and E) step-up and (B, D, and F) step-down. The normal load applied to the silica tip is 10 nN, and the sliding speed is 10 m/s. The topographic height change measured with the center of mass of the counter surface is shown with dashed lines in (A) and (B) on the secondary y axis. The white and gray background areas are the lower and upper terraces, respectively. The snapshots of the shear strain of atoms in the silica and the hydrogen bonds bridging two surfaces at three locations for both step-up and step-down are also shown. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
Reactive MD simulation showing the origins of chemical and physical effects on friction. (A and B) Lateral force, (C and D) shear strain of atoms in the silica where the sign indicates direction relative to sliding, and (E and F) number of hydrogen bonds formed between the graphene step edge and the silica, calculated from simulations as a function of center-of-mass position of the tip with respect to the graphene step edge for (A, C, and E) step-up and (B, D, and F) step-down. The normal load applied to the silica tip is 10 nN, and the sliding speed is 10 m/s. The topographic height change measured with the center of mass of the counter surface is shown with dashed lines in (A) and (B) on the secondary y axis. The white and gray background areas are the lower and upper terraces, respectively. The snapshots of the shear strain of atoms in the silica and the hydrogen bonds bridging two surfaces at three locations for both step-up and step-down are also shown. Credit: Science Advances, doi: 10.1126/sciadv.aaw0513.
The results explained the difficulty of achieving superlubricity on atomically rough surfaces—unless the topographic surface features were chemically inert. In total, the findings suggest the possibility of tuning the COF with prescribed topographic features and prearranged chemical groups. While the concept does not immediately improve industrial applications of friction, it provides fundamental insight to the chemical and topographic origins of friction and therefore holds significant promise for future scientific advances on minimizing resistance at tribological interfaces. Chen et al. envision the work will open possibilities of tunable friction in applied physics.
More information: Zhe Chen et al. Chemical and physical origins of friction on surfaces with atomic steps, Science Advances (2019). DOI: 10.1126/sciadv.aaw0513
Kaiwen Tian et al. Load and Time Dependence of Interfacial Chemical Bond-Induced Friction at the Nanoscale, Physical Review Letters (2017). DOI: 10.1103/PhysRevLett.118.076103
D. Berman et al. Macroscale superlubricity enabled by graphene nanoscroll formation, Science (2015). DOI: 10.1126/science.1262024
Thomas P Senftle et al. The ReaxFF reactive force-field: development, applications and future directions, npj Computational Materials (2016). DOI: 10.1038/npjcompumats.2015.11
© 2019 Science X Network
Citation: Chemical and physical origins of friction on surfaces with atomic steps (2019, August 19) retrieved 5 July 2022 from https://phys.org/news/2019-08-chemical-physical-friction-surfaces-atomic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
