

| Date | 17th, Dec 2019 |
|---|
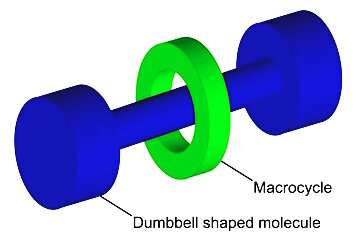 Schematic representation of a rotaxane, the type of molecule that was the subject of study. Credit: Wikimedia Commons
Schematic representation of a rotaxane, the type of molecule that was the subject of study. Credit: Wikimedia Commons
Thanks to a clever chemical design, researchers at the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences (HIMS) have succeeded in making a very fast molecular machine. The moving parts shift more than one nanometer relative to each other in a record-breaking time of 30 billionths of a second. The results were recently published in the Journal of the American Chemical Society.
The molecular machine is a rotaxane, a molecular structure with a ring-shaped molecule around an elongated wire-shaped molecule. The ring can move from one side of the wire to the other, just like a shuttle-bus.
The Amsterdam researchers achieved their record speed thanks to a new molecular design enabling one side of the wire to draw the shuttle towards it, as it were. In addition, they used a whole new concept to trigger the movement, a photochemical acid-base reaction.
Controlling movement with light
The HIMS Molecular Photonics Group has been working for quite some time on rotaxane-based molecular motors of which the movement can be controlled by light. In the case of the simplest rotaxanes, the sliding ring does not have a preferred direction, so it moves randomly over the wire. In more advanced varieties, the wire contains molecular "stations" that introduce a preference of the ring for certain locations on the wire. By chemically changing these stations with the help of light, tuning their attraction to the ring, it is possible to make the ring move from one station to another. In this way, a flash of light of the right color can control movement on a nanometer length scale.
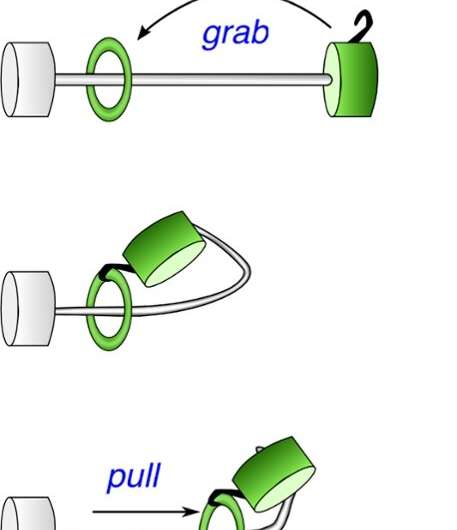 Schematic drawing of the working of the 'harpoon effect'. Credit: HIMS / Maximilian Paradiz
Schematic drawing of the working of the 'harpoon effect'. Credit: HIMS / Maximilian Paradiz
This principle has been successfully applied by the Amsterdam group and elsewhere (e.g. the research groups of Nobel laureates Fraser Stoddart, Jean-Pierre Sauvage and Ben Feringa). At the moment, the field of research of molecular machines is still in its infancy, but potential future applications of such switchable molecular motors are, for example, molecular computers.
The only problem with the mechanism is travel time. If the ring is at a certain station, and another station is made more attractive by means of light, you just have to wait until the ring spontaneously leaves its starting station, and then ends up at the stronger binding station by a random walk over the wire. If the wire is long, this process can take a long time.
Harpoon mechanism
Fred Brouwer and his Ph.D. student Tatu Kumpulainen came up with a solution: they designed a molecular machine in which the terminal station has such a strong attraction to the ring that it deforms the wire allowing the station to grab the ring, and then drag it over the wire to its final destination (see picture). This so-called harpoon mechanism enabled them to create a record speed molecular shuttle. The molecules were made by a master of organic chemistry: Bert Bakker. He has been retired for a long time, but still enjoys his laboratory work.
In order to measure the speed of the molecular shuttle, Brouwer and Kumpulainen worked together with colleagues Matthijs Panman and Sander Woutersen. They used a short pulse of ultraviolet light to induce movement of the ring, and then a second pulse of infrared light to follow its motion. The measured record time was 30 nanoseconds for a covered distance of one nanometer. This means an average speed of 3 cm per second. That may seem slow, but it is 4000 times faster than the fastest biological motor protein (myosin, which causes the contraction of our muscles). One of the challenges for the future is to make the small artificial motor molecules work together just like the motor proteins in our muscles.
More information: Tatu Kumpulainen et al. Accelerating the Shuttling in Hydrogen-Bonded Rotaxanes: Active Role of the Axle and the End Station, Journal of the American Chemical Society (2019). DOI: 10.1021/jacs.9b10005
Citation: Researchers make world's fastest molecular shuttle (2019, December 17) retrieved 5 July 2022 from https://phys.org/news/2019-12-world-fastest-molecular-shuttle.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
