Mar 26, 2020
(Nanowerk Spotlight) In the field of polymer science, the freeze-thaw technique has been used to create physical crosslinks in concentrated solutions by re-organizing the arrangement of polymer chains, thus yielding the corresponding hydrogels. A variant of this method (i.e., freeze casting) requires freezing a concentrated aqueous solutions of carbons and ceramics, which leads to an ordered network by templating in situ produced ice crystals, thus acquiring a hierarchical structured aerogel after drying in vacuum .
However, the prerequisite for both cases is a highly concentrated solution with as little impurities as possible.
By utilizing an unconventional self-healing property observed in noble metal gels, scientists from Technical University Dresden have harmonized the freeze-thaw and freeze-casting process by starting from a dilute aqueous solution of metal nanoparticles, where the concentration was as low as 0.5 mM, which is 2∼3 orders of magnitude lower than in conventional processes.
Moreover, the method fits various noble metals, and multi-scale structures are obtained across 10-9 to 10-5 m, attributed to the synergy of intrinsic stacking of networks and ice shaping effect, facilitating high performance photoelectrocatalysis towards electro-oxidation of ethanol (i.e., the anode reaction for direct ethanol fuel cells).
The team reported their findings in Angewandte Chemie International Edition ("Freeze-Thaw-Promoted Fabrication of Clean and Hierarchically-Structured Noble Metal Gels for Electrocatalysis and Photoelectrocatalysis").
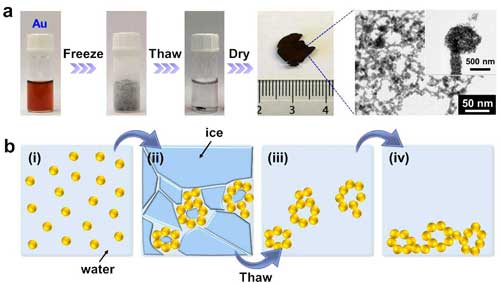 (a) Photographs of the fabrication process and the transmission electron microscopy images for the noble metal aerogels. (b) Schematic illustration of the fabrication process. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
The team derived a clean and multiscale noble metal aerogel structure without addition of any initiators in a 3-step process, as Ran Du, first author of the paper,explains to Nanowerk:
"First, upon freezing of the nanoparticle solution, an array of small and separate gel networks were formed due to dramatically increased ionic strength and nanoparticle concentrations. Meanwhile, the networks were shaped at micrometer scale by generated ice crystals. Second, upon thawing, those small gel pieces sediment to the bottom driven by gravity, touching and fusing together to form a hydrogel. Third, the hydrogel was purified by washing with water, and converted to an aerogel by freeze drying."
Noble metal aerogels are widely investigated for electrocatalysis applications due to their large specific surface areas and the high catalytic activity of noble metals (e.g., palladium and platinum).
However, the fabrication methods suffer from tedious procedures, long preparation times, unavoidable impurities, and uncontrolled multiscale structures, discouraging their fundamental and application-orientated studies.
In the current research, the authors from TU Dresden observed a 40.2% enhancement of catalytic current density for gold palladium (Au-Pd) aerogels upon irradiation by a LED (at a light power density of 133.6 mW cm-2), which is 6.5 times higher than commercial palladium-on-carbon (Pd/C) catalysts.
(a) Photographs of the fabrication process and the transmission electron microscopy images for the noble metal aerogels. (b) Schematic illustration of the fabrication process. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
The team derived a clean and multiscale noble metal aerogel structure without addition of any initiators in a 3-step process, as Ran Du, first author of the paper,explains to Nanowerk:
"First, upon freezing of the nanoparticle solution, an array of small and separate gel networks were formed due to dramatically increased ionic strength and nanoparticle concentrations. Meanwhile, the networks were shaped at micrometer scale by generated ice crystals. Second, upon thawing, those small gel pieces sediment to the bottom driven by gravity, touching and fusing together to form a hydrogel. Third, the hydrogel was purified by washing with water, and converted to an aerogel by freeze drying."
Noble metal aerogels are widely investigated for electrocatalysis applications due to their large specific surface areas and the high catalytic activity of noble metals (e.g., palladium and platinum).
However, the fabrication methods suffer from tedious procedures, long preparation times, unavoidable impurities, and uncontrolled multiscale structures, discouraging their fundamental and application-orientated studies.
In the current research, the authors from TU Dresden observed a 40.2% enhancement of catalytic current density for gold palladium (Au-Pd) aerogels upon irradiation by a LED (at a light power density of 133.6 mW cm-2), which is 6.5 times higher than commercial palladium-on-carbon (Pd/C) catalysts.
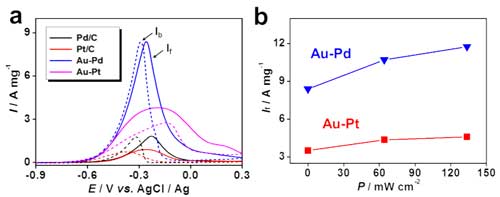 (a) Electro-oxidation of ethanol by using commercial Pd/C, commercial Pt/C, the Au-Pd aerogel, and the Au -Pt aerogel in dark conditions. The solid and dashed lines represent the forward and backward scans, respectively. (b) The dependence of catalytic current densities of aerogel catalysts on the power density of the light. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
As mentioned above, various synthesis challenges in the field of noble metal aerogels have discouraged the evaluation of the intrinsic properties as well as the optimization of the application performance of noble metal aerogels.
In this report, the authors overcome these challenges for creating clean and hierarchically structured noble metal aerogels, and demonstrate impressive photoelectrocatalytic performance of these materials.
Du concludes that their strategy may further the fabrication of widespread clean and multi-level structured hydrogels, aerogels, and foams for specific applications.
By
Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk
(a) Electro-oxidation of ethanol by using commercial Pd/C, commercial Pt/C, the Au-Pd aerogel, and the Au -Pt aerogel in dark conditions. The solid and dashed lines represent the forward and backward scans, respectively. (b) The dependence of catalytic current densities of aerogel catalysts on the power density of the light. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
As mentioned above, various synthesis challenges in the field of noble metal aerogels have discouraged the evaluation of the intrinsic properties as well as the optimization of the application performance of noble metal aerogels.
In this report, the authors overcome these challenges for creating clean and hierarchically structured noble metal aerogels, and demonstrate impressive photoelectrocatalytic performance of these materials.
Du concludes that their strategy may further the fabrication of widespread clean and multi-level structured hydrogels, aerogels, and foams for specific applications.
By
Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk
 (a) Photographs of the fabrication process and the transmission electron microscopy images for the noble metal aerogels. (b) Schematic illustration of the fabrication process. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
The team derived a clean and multiscale noble metal aerogel structure without addition of any initiators in a 3-step process, as Ran Du, first author of the paper,explains to Nanowerk:
"First, upon freezing of the nanoparticle solution, an array of small and separate gel networks were formed due to dramatically increased ionic strength and nanoparticle concentrations. Meanwhile, the networks were shaped at micrometer scale by generated ice crystals. Second, upon thawing, those small gel pieces sediment to the bottom driven by gravity, touching and fusing together to form a hydrogel. Third, the hydrogel was purified by washing with water, and converted to an aerogel by freeze drying."
Noble metal aerogels are widely investigated for electrocatalysis applications due to their large specific surface areas and the high catalytic activity of noble metals (e.g., palladium and platinum).
However, the fabrication methods suffer from tedious procedures, long preparation times, unavoidable impurities, and uncontrolled multiscale structures, discouraging their fundamental and application-orientated studies.
In the current research, the authors from TU Dresden observed a 40.2% enhancement of catalytic current density for gold palladium (Au-Pd) aerogels upon irradiation by a LED (at a light power density of 133.6 mW cm-2), which is 6.5 times higher than commercial palladium-on-carbon (Pd/C) catalysts.
(a) Photographs of the fabrication process and the transmission electron microscopy images for the noble metal aerogels. (b) Schematic illustration of the fabrication process. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
The team derived a clean and multiscale noble metal aerogel structure without addition of any initiators in a 3-step process, as Ran Du, first author of the paper,explains to Nanowerk:
"First, upon freezing of the nanoparticle solution, an array of small and separate gel networks were formed due to dramatically increased ionic strength and nanoparticle concentrations. Meanwhile, the networks were shaped at micrometer scale by generated ice crystals. Second, upon thawing, those small gel pieces sediment to the bottom driven by gravity, touching and fusing together to form a hydrogel. Third, the hydrogel was purified by washing with water, and converted to an aerogel by freeze drying."
Noble metal aerogels are widely investigated for electrocatalysis applications due to their large specific surface areas and the high catalytic activity of noble metals (e.g., palladium and platinum).
However, the fabrication methods suffer from tedious procedures, long preparation times, unavoidable impurities, and uncontrolled multiscale structures, discouraging their fundamental and application-orientated studies.
In the current research, the authors from TU Dresden observed a 40.2% enhancement of catalytic current density for gold palladium (Au-Pd) aerogels upon irradiation by a LED (at a light power density of 133.6 mW cm-2), which is 6.5 times higher than commercial palladium-on-carbon (Pd/C) catalysts.
 (a) Electro-oxidation of ethanol by using commercial Pd/C, commercial Pt/C, the Au-Pd aerogel, and the Au -Pt aerogel in dark conditions. The solid and dashed lines represent the forward and backward scans, respectively. (b) The dependence of catalytic current densities of aerogel catalysts on the power density of the light. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
As mentioned above, various synthesis challenges in the field of noble metal aerogels have discouraged the evaluation of the intrinsic properties as well as the optimization of the application performance of noble metal aerogels.
In this report, the authors overcome these challenges for creating clean and hierarchically structured noble metal aerogels, and demonstrate impressive photoelectrocatalytic performance of these materials.
Du concludes that their strategy may further the fabrication of widespread clean and multi-level structured hydrogels, aerogels, and foams for specific applications.
By
Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk
(a) Electro-oxidation of ethanol by using commercial Pd/C, commercial Pt/C, the Au-Pd aerogel, and the Au -Pt aerogel in dark conditions. The solid and dashed lines represent the forward and backward scans, respectively. (b) The dependence of catalytic current densities of aerogel catalysts on the power density of the light. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge)
As mentioned above, various synthesis challenges in the field of noble metal aerogels have discouraged the evaluation of the intrinsic properties as well as the optimization of the application performance of noble metal aerogels.
In this report, the authors overcome these challenges for creating clean and hierarchically structured noble metal aerogels, and demonstrate impressive photoelectrocatalytic performance of these materials.
Du concludes that their strategy may further the fabrication of widespread clean and multi-level structured hydrogels, aerogels, and foams for specific applications.
By
Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk
Nanowerk Newsletter
Get our Nanotechnology Spotlight updates to your inbox!
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.

