

| Date | 24th, Apr 2020 |
|---|
![Design and sensing mechanism of the K+ nanosensor. (A) Schematic illustration for the synthesis of the nanosensor. The NaYF4:Yb/Tm@NaYF4:Yb/Nd (UCNP) core was synthesized and coated with a dense silica layer and a successive mesoporous silica shell. Etching away of the dense silica layer forms a hollow cavity that allows the loading of PBFI. The nanosensor was lastly coated with the K+-selective filter membrane. (B) Schematics showing a magnified view of the nanosensor [from the red dotted box in (A)] and its K+ sensing mechanism. The filter membrane layer allows only K+ to diffuse into and out of the nanosensor, thus excluding the interference from other cations. Once diffused into the nanosensor, K+ will bind to PBFI immediately. Upon NIR irradiation, the upconverted UV light from the UCNPs excites PBFI, leading to the emission of K+-bonded PBFI. Credit: Science Advances, doi: 10.1126/sciadv.aax9757 Highly sensitive nanosensor detects subtle potassium changes in the brain](https://scx1.b-cdn.net/csz/news/800a/2020/2-highlysensit.jpg) Design and sensing mechanism of the K+ nanosensor. (A) Schematic illustration for the synthesis of the nanosensor. The NaYF4:Yb/Tm@NaYF4:Yb/Nd (UCNP) core was synthesized and coated with a dense silica layer and a successive mesoporous silica shell. Etching away of the dense silica layer forms a hollow cavity that allows the loading of PBFI. The nanosensor was lastly coated with the K+-selective filter membrane. (B) Schematics showing a magnified view of the nanosensor [from the red dotted box in (A)] and its K+ sensing mechanism. The filter membrane layer allows only K+ to diffuse into and out of the nanosensor, thus excluding the interference from other cations. Once diffused into the nanosensor, K+ will bind to PBFI immediately. Upon NIR irradiation, the upconverted UV light from the UCNPs excites PBFI, leading to the emission of K+-bonded PBFI. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Design and sensing mechanism of the K+ nanosensor. (A) Schematic illustration for the synthesis of the nanosensor. The NaYF4:Yb/Tm@NaYF4:Yb/Nd (UCNP) core was synthesized and coated with a dense silica layer and a successive mesoporous silica shell. Etching away of the dense silica layer forms a hollow cavity that allows the loading of PBFI. The nanosensor was lastly coated with the K+-selective filter membrane. (B) Schematics showing a magnified view of the nanosensor [from the red dotted box in (A)] and its K+ sensing mechanism. The filter membrane layer allows only K+ to diffuse into and out of the nanosensor, thus excluding the interference from other cations. Once diffused into the nanosensor, K+ will bind to PBFI immediately. Upon NIR irradiation, the upconverted UV light from the UCNPs excites PBFI, leading to the emission of K+-bonded PBFI. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Researchers have developed a number of potassium ion (K+) probes to detect fluctuating K+ concentrations during a variety of biological processes. However, such probes are not sensitive enough to detect physiological fluctuations in living animals and it is not easy to monitor deep tissues with short-wavelength excitations that are in use so far. In a new report, Jianan Liu and a team of researchers in neuroscience, chemistry, and molecular engineering in China, describe a highly sensitive and selective nanosensor for near infrared (NIR) K+ ion imaging in living cells and animals. The team constructed the nanosensor by encapsulating upconversion nanoparticles (UCNPs) and a commercial potassium ion indicator in the hollow cavity of mesoporous silica nanoparticles and coated them with a K+ selective filter membrane. The membrane adsorbed K+ from the medium and filtered away any interfering cations. In its mechanism of action, UCNPs converted NIR to ultraviolet (UV) light to excite the potassium ion indicator and detect fluctuating potassium ion concentrations in cultured cells and in animal models of disease including mice and zebrafish larvae. The results are now published on Science Advances.
The most abundant intracellular cation potassium (K+) is extremely crucial in a variety of biological processes including neural transmission, heartbeat, muscle contraction and kidney function. Variations in the intracellular or extracellular K+ concentration (referred herein as [K+]) suggest abnormal physiological functions including heart dysfunction, cancer, and diabetes. As a result, researchers are keen to develop effective strategies to monitor the dynamics of [K+] fluctuations, specifically with direct optical imaging.
Most existing probes are not sensitive to K+ detection under physiological conditions and cannot differentiate fluctuations between [K+] and the accompanying sodium ion ([Na+]) during transmembrane transport in the Na+/K+ pumps. While fluorescence lifetime imaging can distinguish K+ and Na+ in water solution, the method requires specialized instruments. Most K+ sensors are also activated with short wavelength light including ultraviolet (UV) or visible light—leading to significant scattering and limited penetration depth when examining living tissues. In contrast, the proposed near-infrared (NIR) imaging technique will offer unique advantages during deep tissue imaging as a plausible alternative.
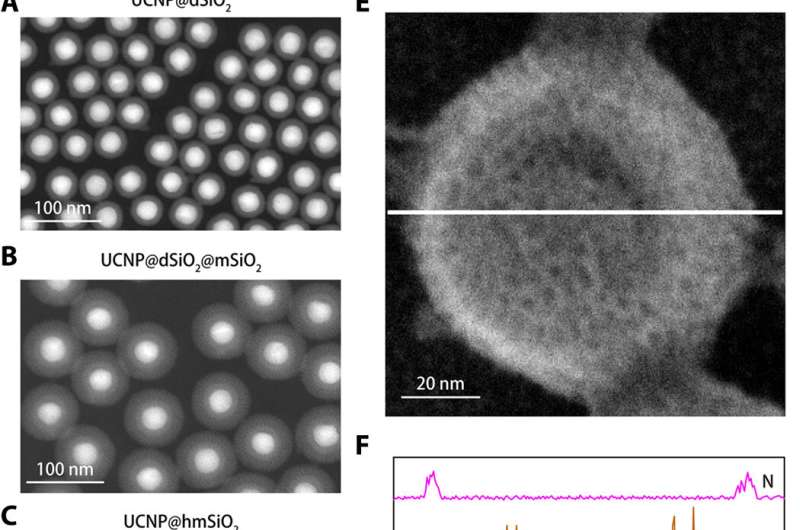 Structural characterization of the K+ nanosensor. (A to C) High-angle annular dark-field images of UCNP@dSiO2 (A), UCNP@dSiO2@mSiO2 (B), and UCNP@hmSiO2 (C). (D) Scanning electron microscopy (SEM) image of the shielded nanosensor. (E) SEM image of the shielded nanosensor immersed in an aqueous solution containing 150 mM Na+, 150 mM K+, 2 mM Ca2+, 2 mM Mg2+, 50 μM Fe2+, 2 mM Zn2+, 50 μM Mn2+, and 50 μM Cu2+. (F) EDS elemental line scanning profiles along the white line in (E) reveal that only K+ signals are present in the mesopores and hollow cavities of the shielded nanosensors. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Structural characterization of the K+ nanosensor. (A to C) High-angle annular dark-field images of UCNP@dSiO2 (A), UCNP@dSiO2@mSiO2 (B), and UCNP@hmSiO2 (C). (D) Scanning electron microscopy (SEM) image of the shielded nanosensor. (E) SEM image of the shielded nanosensor immersed in an aqueous solution containing 150 mM Na+, 150 mM K+, 2 mM Ca2+, 2 mM Mg2+, 50 μM Fe2+, 2 mM Zn2+, 50 μM Mn2+, and 50 μM Cu2+. (F) EDS elemental line scanning profiles along the white line in (E) reveal that only K+ signals are present in the mesopores and hollow cavities of the shielded nanosensors. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Designing the K+ nanosensor and characterizing its structure
To engineer the nanosensor, Liu et al. encapsulated upconversion nanoparticles (UCNPs) and a commercial K+ indicator—potassium-binding benzofuran isophthalate (PBFI) into the core of mesoporous silica nanoparticles (MSNs). The UCNPs were able to convert NIR light into UV light and excite the acceptor of the K+ indicator through luminescence resonance energy transfer. They shielded the outer surface of silica nanoparticles with a thin layer of K+ selective filter membrane with micropores created from carbonyl oxygen for specificity. The setup favored the free transfer of K+ through the membrane pore, while preventing other biologically relevant cations from diffusing through. The technique allowed them to detect slight fluctuations in [K+] in the solution. The team used transmission electron microscopy (TEM) to observe the well-controlled structure and appearance of the nanoparticles during each step of nanosensor construction. Dynamic light scattering confirmed the presence of a filter membrane on the surface of the shielded nanosensor.
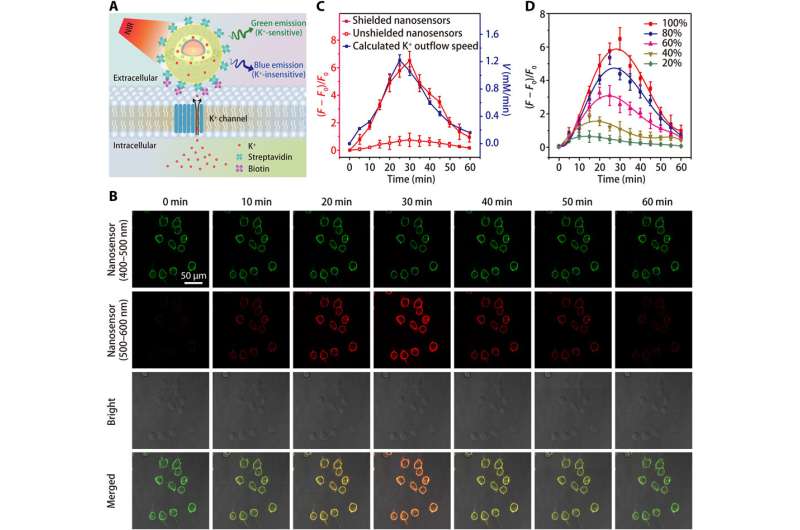 Imaging of K+ efflux in HEK 293 cells. (A) Schematics showing the detection of K+ efflux by a streptavidin-conjugated nanosensor, which is tethered to a biotin-modified cell. (B) Confocal microscopy images showing the fluorescence (at 400 to 500 nm and 500 to 600 nm) of nanosensor-labeled HEK 293 cells at different time points after treatment with the K+ efflux stimulator (a mixture of 5 μM nigericin, 5 μM bumetanide, and 10 μM ouabain). (C) Time courses of nanosensor fluorescence variations and calculated time dependence of K+ efflux rate after treatment with K+ efflux stimulator. (D) Time-dependent fluorescence fluctuations of shielded nanosensor-labeled HEK 293 cells after treatments with different concentrations (20, 40, 60, 80, and 100%) of K+ efflux stimulator. Results from five independent experiments were summarized as mean ± SEM in (C) and (D). Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Imaging of K+ efflux in HEK 293 cells. (A) Schematics showing the detection of K+ efflux by a streptavidin-conjugated nanosensor, which is tethered to a biotin-modified cell. (B) Confocal microscopy images showing the fluorescence (at 400 to 500 nm and 500 to 600 nm) of nanosensor-labeled HEK 293 cells at different time points after treatment with the K+ efflux stimulator (a mixture of 5 μM nigericin, 5 μM bumetanide, and 10 μM ouabain). (C) Time courses of nanosensor fluorescence variations and calculated time dependence of K+ efflux rate after treatment with K+ efflux stimulator. (D) Time-dependent fluorescence fluctuations of shielded nanosensor-labeled HEK 293 cells after treatments with different concentrations (20, 40, 60, 80, and 100%) of K+ efflux stimulator. Results from five independent experiments were summarized as mean ± SEM in (C) and (D). Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Performance of the nanosensor in water and during [K+] fluctuations in cells.
The team tested the enhanced sensitivity of the shielded nanosensor in a physiological range (0 to 150 mM) and showed a 12-fold increase in fluorescence intensity compared to unshielded nanosensors. The K+ probes had to display high selectivity against Na+, which Liu et al. verified using the shielded nanosensor by rapidly detecting consistent fluorescence sensitivity to fluctuating [K+], while remaining unaffected by increasing [Na+].
Since living cells rely on the sodium-potassium adenosine triphosphatase (Na+/K+ pump) to maintain a steep [K+] gradient across their plasma membrane, the process is partially responsible for the cell's energy expenditure. Defects in cellular energy metabolism can lead to a loss of the [K+] gradient, while giving rise to extracellular [K+] known as [K+]0, which the scientists monitored to obtain a valuable indicator of cell viability and growth. Thereafter, they increased the specificity of the nanosensor to detect cell death or proliferation rates by grafting polyethylene glycol (PEG) on the surface of nanosensors in a culture medium containing the human embryonic kidney 293 cell line. They then optimized the protocol by anchoring large numbers of nanosensors onto cell membranes using streptavidin-conjugated nanosensors to biotin-modified cells. The results highlighted improved sensitivity of shielded nanosensors to continuously monitor the K+ efflux.
 K+ imaging results of the shielded nanosensors-treated mouse brain upon initiating spreading depression by KCl triggering. The full-length video is eight times faster than the real speed. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
K+ imaging results of the shielded nanosensors-treated mouse brain upon initiating spreading depression by KCl triggering. The full-length video is eight times faster than the real speed. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Spreading waves in the mouse intact brain
The team then applied the shielded nanosensor to investigate cortical spreading depression (CSD) in the mouse brain as a wave-like propagation of neural activity. The process typically involves a slow propagation release of K+ in the cortical surface and could be triggered in the mouse brain via potassium chloride (KCl) incubation. The scientists simultaneously monitored the local field potential and optical signal through the surgical cranial window and observed a wave of increasing [K+]0 propagate gradually across the cortex after stimulation. Liu et al. did not observe a wave in mice injected with unshielded nanosensors, indicating the importance of the outer filter for improved sensitivity of the nanosensor. The recorded wave velocity did not vary significantly from the values obtained using blood oxygen-level dependent magnetic resonance imaging (MRI) in patients with migraine aura.
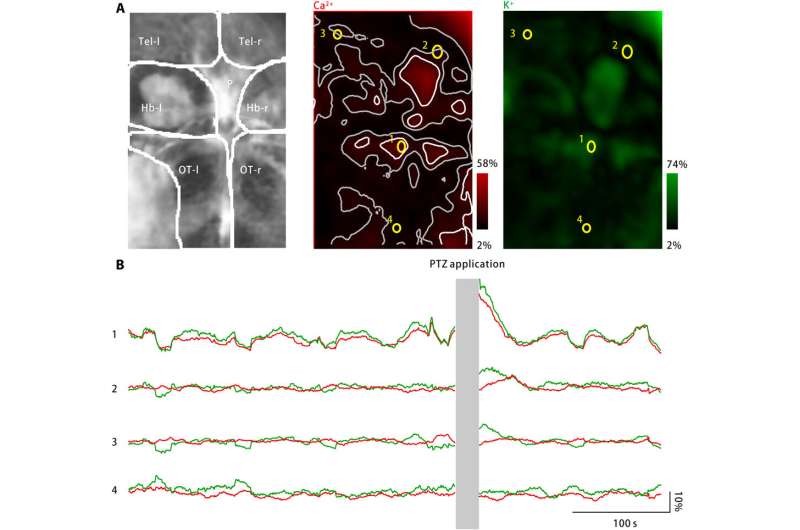 Extracellular potassium burst in larval zebrafish brain upon PTZ treatment. (A) PTZ treatment induced increases in both neuronal calcium activity (middle) and extracellular potassium concentration (right). Left: Imaged brain areas include the left and right telencephala (Tel-l and Tel-r, respectively), the left and right habenulae (Hb-l and Hb-r, respectively), the pineal body (P), and the left and right optic tecta (OT-l and OT-r, respectively). Middle: Neuronal calcium activity was monitored by using a genetically expressed calcium indicator, jRGECO1a. The measured response amplitude is coded in red and mapped back to the imaged brain region. Scattered activity spots are marked as white, and their neighboring zones are marked as gray. Four ROIs (yellow) are selected. Right: Extracellular potassium concentration was monitored by using the potassium nanosensor. The measured response amplitude is coded in green and mapped back to the imaged brain region. (B) Neuronal calcium activity (red) and extracellular potassium concentrations observed for the four representative ROIs are marked in (A). After PTZ application, both neuronal calcium activity and extracellular potassium concentration have increased at several activity spots, including the pineal body and the anterior optic tecta, as represented by ROI1. At neighboring zones of the activity spots, neuronal calcium activity change is absent or minimal, while the extracellular potassium concentration continues to increase (ROI2 and ROI3). However, in the area far from the activity spots (ROI4), neither neuronal calcium activity nor extracellular potassium concentration has increased. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
Extracellular potassium burst in larval zebrafish brain upon PTZ treatment. (A) PTZ treatment induced increases in both neuronal calcium activity (middle) and extracellular potassium concentration (right). Left: Imaged brain areas include the left and right telencephala (Tel-l and Tel-r, respectively), the left and right habenulae (Hb-l and Hb-r, respectively), the pineal body (P), and the left and right optic tecta (OT-l and OT-r, respectively). Middle: Neuronal calcium activity was monitored by using a genetically expressed calcium indicator, jRGECO1a. The measured response amplitude is coded in red and mapped back to the imaged brain region. Scattered activity spots are marked as white, and their neighboring zones are marked as gray. Four ROIs (yellow) are selected. Right: Extracellular potassium concentration was monitored by using the potassium nanosensor. The measured response amplitude is coded in green and mapped back to the imaged brain region. (B) Neuronal calcium activity (red) and extracellular potassium concentrations observed for the four representative ROIs are marked in (A). After PTZ application, both neuronal calcium activity and extracellular potassium concentration have increased at several activity spots, including the pineal body and the anterior optic tecta, as represented by ROI1. At neighboring zones of the activity spots, neuronal calcium activity change is absent or minimal, while the extracellular potassium concentration continues to increase (ROI2 and ROI3). However, in the area far from the activity spots (ROI4), neither neuronal calcium activity nor extracellular potassium concentration has increased. Credit: Science Advances, doi: 10.1126/sciadv.aax9757
To extend applications of the nanosensor, Liu et al. monitored neuronal calcium levels and extracellular potassium concentrations using zebrafish larvae. While a large increase in the extracellular potassium concentration can cause intense neuronal activation to cause CSD and epilepsy, no direct evidence exists to show changes in extracellular potassium during the disease. The team therefore engineered a disease model using zebrafish larvae to increase extracellular potassium concentrations and observed disease characteristic neuronal activation in specific brain regions.
In this way, Jianan Liu and colleagues engineered a potassium ion nanosensor with extremely high sensitivity and selectivity. The external coating of a selective filter membrane enhanced the selectivity, sensitivity, and kinetics of the device for rapid and quantitative [K+] detection in living cells and intact brains. The shielded nanosensor will have broad applications in brain research to improve the understanding of abnormal [K+]-related diseases. The method alongside optical fiber-based endoscope and photometry will allow real-time potassium imaging in freely moving animals.
More information: Jianan Liu et al. A highly sensitive and selective nanosensor for near-infrared potassium imaging, Science Advances (2020). DOI: 10.1126/sciadv.aax9757
Prashant Padmawar et al. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator, Nature Methods (2005). DOI: 10.1038/nmeth801
Michelle L. Gumz et al. An Integrated View of Potassium Homeostasis, New England Journal of Medicine (2015). DOI: 10.1056/NEJMra1313341
© 2020 Science X Network
Citation: Highly sensitive nanosensor detects subtle potassium changes in the brain (2020, April 24) retrieved 28 June 2022 from https://phys.org/news/2020-04-highly-sensitive-nanosensor-subtle-potassium.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
