When the right catalysts are mixed with the right chemical compounds, molecules that would otherwise take years to interact do so in mere seconds. Chemical catalysts are the change agents behind the production of just about everything we use in our daily lives, from plastics to prescription drugs.
The Rochester team, which reviewed close to 600 previous papers involving the use of pulsed lasers in liquids, reported there is a way to shorten that process dramatically — by using pulsed lasers in liquids to quickly create carefully tuned, systematic arrays of nanoparticles that can be easily compared and tested for use as catalysts.
The study was led by Astrid Müller, a professor of chemical engineering at the University of Rochester, along with co-authors Ryland Forsythe, Connor Cox, and Madeleine Wilsey.

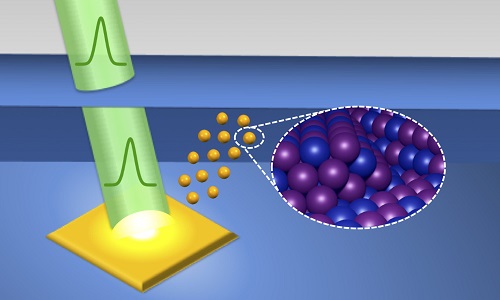
The process begins with a pulsed laser that is directed at a solid material immersed in liquid. This creates a high-temperature, high-pressure plasma near the surface of the solid. As the plasma decays, it vaporizes molecules in the surrounding liquid, leading to a cavitation bubble. Within the bubble, chemical reactions begin to occur between particles from the liquid and particles that were ablated, or knocked loose, from the solid.
After periodic expansions and contractions, the cavitation bubble violently implodes, causing shock waves and rapid cooling. Nanoparticles from the bubble condense in small clusters that are injected into the surrounding liquid and become stable.
The method offers multiple advantages over traditional wet-lab synthesis of nanomaterials, Müller said. Because reactions are confined primarily within the cavitation bubble, the resulting nanoparticles have remarkably uniform properties because they are created in the same conditions. Additionally, the properties of the nanoparticles can easily be fine-tuned by adjusting the laser pulses and the chemical compositions of the solid and surrounding fluid. The laser-made nanocatalysts are also intrinsically more active than those created through wet chemistry methods. Metastable nanomaterials with nonequilibrium structures and composites can easily be produced; such materials can’t be made under moderate temperatures and pressures.
“These advantages make this an indispensable tool for discovery,” Müller said.
Pulsed-laser-in-liquid synthesis has had only limited commercial use. The startup cost of investing in laser technology is a stumbling block for many companies, Müller said. “But that will change as this method gets more and more traction,” she said.
The research was published in Chemical Reviews (www.doi.org/10.1021/acs.chemrev.0c01069).

