A study published in the journal PNAS reports the development of nanosized and metastable molybdenum oxide as efficient negative electrode material for aqueous electrolytes in lithium-ion batteries. High charge density, capacity, and stability are key findings.
Study: Nanosized and metastable molybdenum oxides as negative electrode materials for durable high-energy aqueous Li-ion batteries. Image Credit: Smile Fight/Shutterstock.com
Rechargeable batteries are in huge demand due to the popularity of using portable devices like mobile phones and laptops, to electric vehicles.
Generally, a battery consists of two electrodes, an anode (the reductant) and a cathode (the oxidant), that is separated by an electrolyte that transfers the ionic component of chemical reaction inside the cell. The output of the battery is current at a specific voltage for the time duration depending on the charge stored.
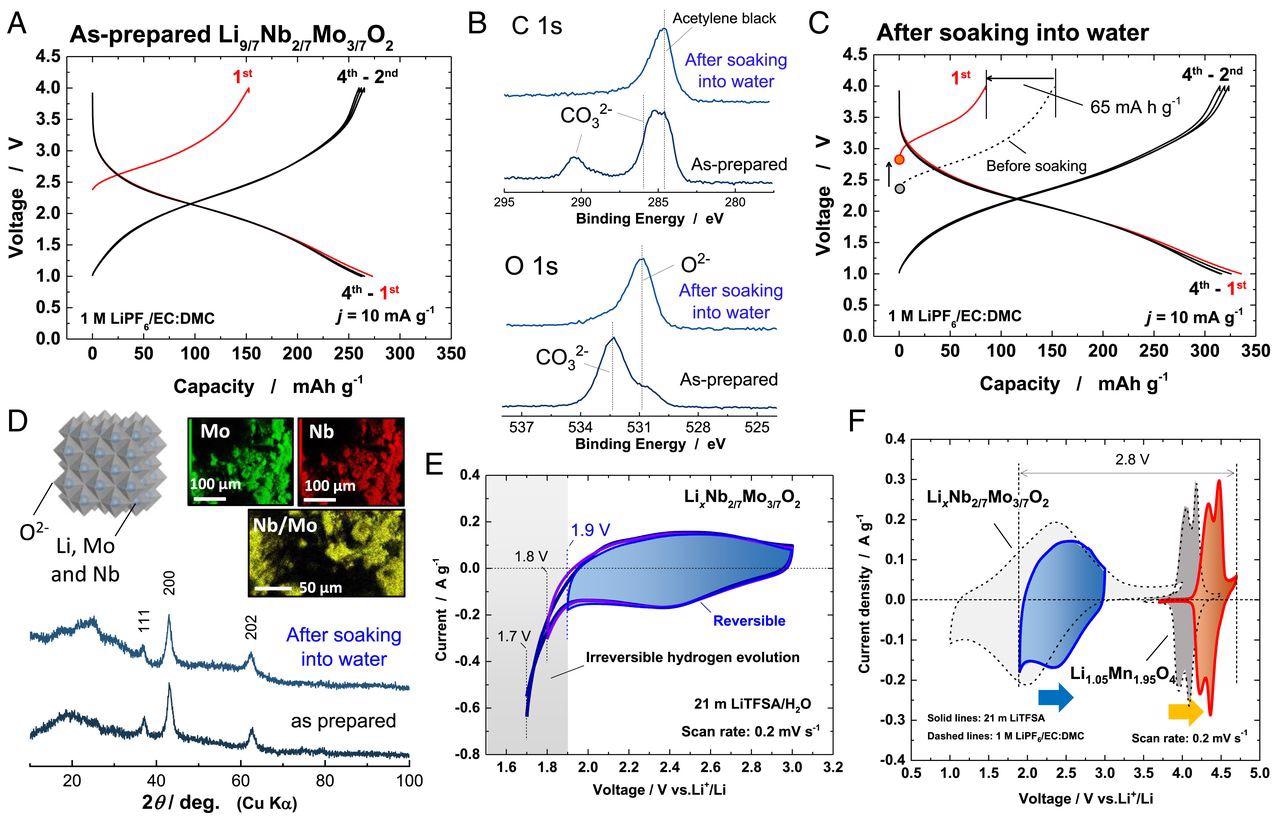
Characterization of LixNb2/7Mo3/7O2. (A) Charge/discharge curves (in a nonaqueous cell) of as-prepared Li9/7Nb2/7Mo3/7O2. (B) SOXPES spectra of C 1s and O 1s core levels of the sample before and after soaking in water. (C) Charge/discharge curves of LixNb2/7Mo3/7O2 after soaking in water. (D) X-ray diffraction (XRD) patterns of the sample before and after soaking in water and energy-dispersive X-ray spectroscopy (EDX) elemental maps of the sample after soaking in water. A schematic illustration of the crystal structure of LixNb2/7Mo3/7O2 drawn using the program VESTA (33) is also shown. (E) Cyclic voltammograms of LixNb2/7Mo3/7O2 in 21 m LiTFSA at a scan rate of 0.2 mV ⋅ s−1. A blue vertical line shows the lowest potential limit available in 21 m LiTFSA aqueous electrolyte. (F) Cyclic voltammograms of Li1.05Mn1.95O4 and LixNb2/7Mo3/7O2 in 21 m LiTFSA (solid lines) and 1 M LiPF6/EC:DMC (dashed lines), respectively. Image Credit: Suo, L., et al.
Lithium Ion Batteries
Currently, lithium-ion batteries (LIBs) are considered best among rechargeable batteries due to their higher energy density and efficiency.
LIBs used for electric vehicles have 50 kWh battery power, which can provide energy for a 300 km drive. Moreover, grid-scale energy storage systems require battery power on a scale of megawatt-hour to gigawatt-hour.
However, due to the use of flammable organic electrolytes, large-scale production of LIBs raises safety issues. A potential method of resolving these issues involves the use of aqueous electrolytes, which have other benefits such as higher ionic conductivity and environmental safety.
The use of aqueous electrolytes results in few demerits in LIBs compared to organic electrolytes, one of them is the lower energy density due to low operating voltage of LIBs with aqueous electrolytes.
This condition arises due to the slow kinetics of water electrolysis resulting in a narrow electrochemical stability window of aqueous electrolytes. The operation voltage window of aqueous electrolytes is typically