Due to their harmful and toxic impacts on many living things, metal ions such as chromium (VI) in industrial wastewater are becoming a major environmental concern. A recent study published in the journal Scientific Reports tackles this problem by creating low-cost and eco-friendly nanoparticles using waste from the drinkable water sector for the efficient removal of Cr (VI) from industrial wastewater.
Study: Optimization and mechanisms of rapid adsorptive removal of chromium (VI) from wastewater using industrial waste derived nanoparticles. Image Credit: pingphuket/Shutterstock.com
Why is Chromium (VI) So Dangerous?
The release of toxic metals from industrial wastewater into the marine ecosystem is causing growing worldwide concern since these hazardous elements can have substantial detrimental consequences on the environment and public health. Chromium and its derivatives are among the most hazardous elements present in industrial wastewater.
The harmful byproducts from many industries contain chromium compounds, including the leather industry, mining, textiles, electroplating, and chemical sectors.
Chromium ions exist in the water habitats as chromium III (Trivalent) and chromium VI (Hexavalent). Although Cr (III) is an important macronutrient in humans, Cr (VI) is 100x more hazardous than its trivalent counterpart owing to its high oxidative activity and ease of absorption through cellular membranes.
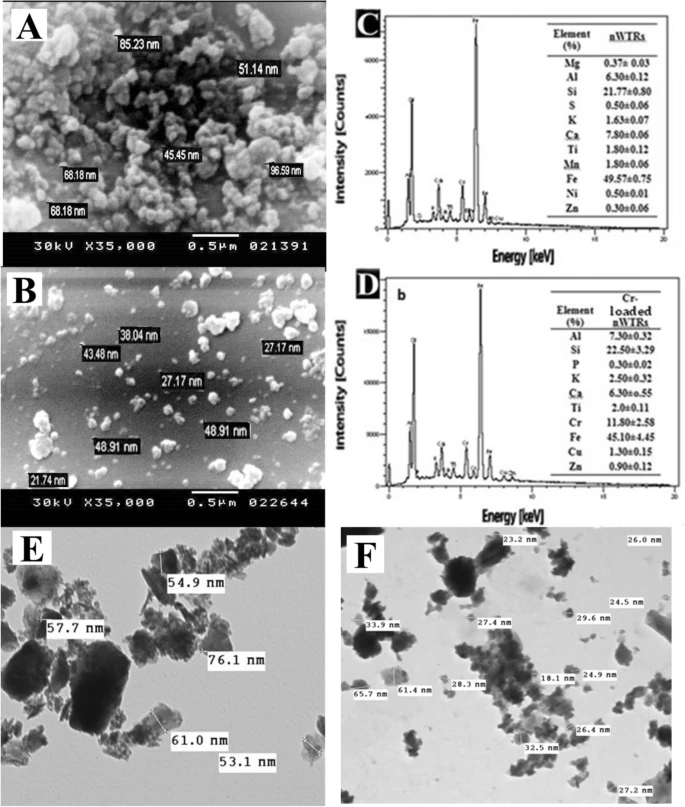
Figure 1. Scanning electron microscopy (SEM) image and energy dispersive X-ray (EDX) elemental distribution of nWTRs (A–C), the Cr(VI)—loaded nWTRs (B–D), Transmission electron microscopy (TEM) image of nWTRs (E) and Cr(VI)—loaded nWTRs (F). © Hamadeen, H. M., et al. (2022).
Leather Industry: A Major Source of Chromium Pollution
The international leather marketplace is rapidly expanding, but the sector has a significant negative ecological effect due to poisonous chromium (VI) used in the tanning process. As a result, the leather industry is regarded as one of the world's major contributors to Cr (VI) pollution.
Chromium-based manufacturing technology is used for tanning about 85% of all leather goods due to its low prices, color consistency, quick processing, and heat resilience. To inhibit water from reaching the pores in the skin and triggering rot, the tanning procedure incorporates chromium salts with collagen ingredients.
The skin absorbs 60 to 80% of the chromium administered during the tanning phase, and the remaining is typically discharged via the sewage system in the form of industrial wastewater, severely harming aquatic environments.
Current Cr (VI) Removal Methods and Their Limitations
Many approaches, including nanoparticle-based treatment, absorption, ion exchange, advanced oxidation techniques, and chemical coagulation, are currently employed to remove chromium (VI) from industrial wastewater.
However, these procedures have inherent drawbacks, including poor cost effectiveness, high labor requirements, increased time consumption, sludge creation that creates disposal issues, and the generation of secondary pollutants. These constraints substantially restrict the practical application of these techniques in industrial settings.
As a result, developing a green, low-cost, and effective technology for removing metals and other contaminants from industrial wastewater while minimizing unwanted impacts on the treatment system is a critical need of the hour.
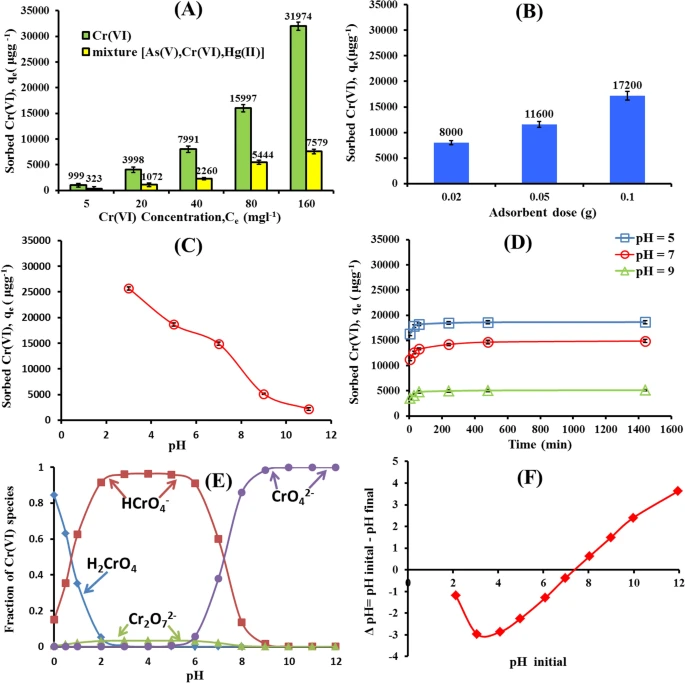
Figure 2. Effect of initial Cr(VI) concentration and competing ions (A), adsorbent dose (B) solution pH (C) and Contact Time (D) on the Cr(VI) adsorption by nWTRs. Cr(VI) species (E), and zero point charage of nWTRs (F). © Hamadeen, H. M., et al. (2022).
Water Treatment Residual-based Nanoparticles for Cr (VI) Removal
Water treatment residual (WTR) is a waste material of the consumable water treatment industry that is produced when aluminum sulfate is employed in the flocculation process. Every year, thousands of tons of WTRs are produced worldwide, posing an environmental and economic challenge.
Using WTRs as effective and affordable nanomaterials-based adsorbents for heavy metal clean-up in industrial wastewater is an appealing remediation method. As a result, using WTR-based nanoparticles as green adsorbents may significantly improve the long-term treatment of Cr (VI) from industrial wastewater.
The use of nanoparticles in industrial wastewater treatment has recently gained prominence due to their improved performance over bulk equivalents owing to unique physicochemical properties such as compact diameters and high adsorption capabilities.
Highlights and Key Developments of the Current Study
In this study, the researchers devised a simple and cost-effective technique for converting drinking water industry byproducts (WTRs) into beneficial WTR-based nanoparticles for increased removal of Cr (VI) from industrial wastewater. This research also included the mineralogical and morphological characterization, as well as the compositions of WTR-based nanoparticles.
The as-prepared WTR-based nanoparticles were shown to be very effective in removing Cr (VI) from industrial wastewater. The researchers' linearized Langmuir model was significantly better than the other models and demonstrated an exceptional capacity to describe Cr (VI) adsorption data using WTR-based nanoparticles.
The highest adsorption rate of WTR-based nanoparticles for Cr (VI) was predicted to be 15 times greater than the maximum absorption rate of bulk WTRs. WTR-based nanoparticles removed Cr (VI) from industrial wastewater effluents with 98.12% and 96.86% efficiency using batch and column procedures, respectively.
Thus, replacing costly commercial bulk adsorbents with low-cost WTR-based nanoparticles (produced from industrial waste products) demonstrates a novel approach to industrial wastewater treatment. Because of their sustainability, availability, and minimal environmental imprint, these WTR-based nanoparticles are expected to be employed as next-generation Cr (VI) adsorbents.
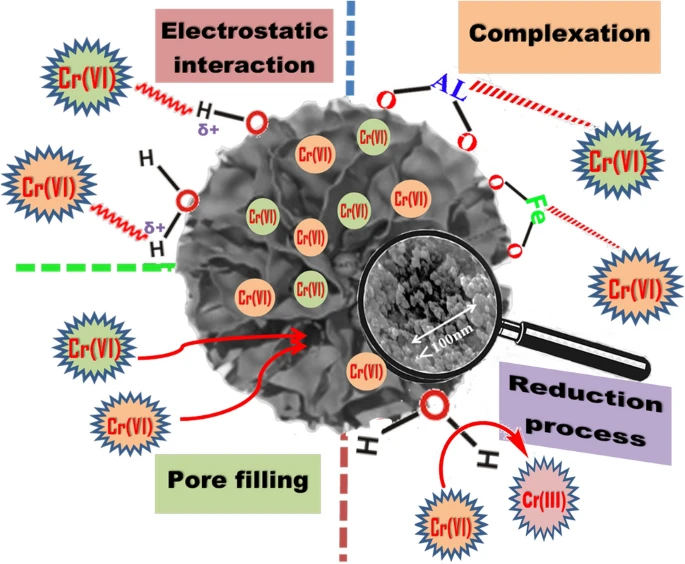
Figure 3. Schematic representation of Cr(VI) removal mechanism by nWTRs. © Hamadeen, H. M., et al. (2022).
Reference
Hamadeen, H. M., et al. (2022). Optimization and mechanisms of rapid adsorptive removal of chromium (VI) from wastewater using industrial waste-derived nanoparticles. Scientific Reports. Available at: https://www.nature.com/articles/s41598-022-18494-0
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.