Despite the efficiency of chemotherapy in treating primary tumors, it may induce undesirable metastasis because of proinflammatory factors being released from impaired cells, upregulating inflammatory reactions.
Study: Therapeutic Nanocarriers Inhibit Chemotherapy-Induced Breast Cancer Metastasis. Image Credit: Design_Cells/Shutterstock.com
An article published in Advanced Science presented polymeric nanoparticles that can concurrently scavenge proinflammatory factors and deliver chemotherapeutics to inhibit chemotherapy-induced metastatic breast cancer.
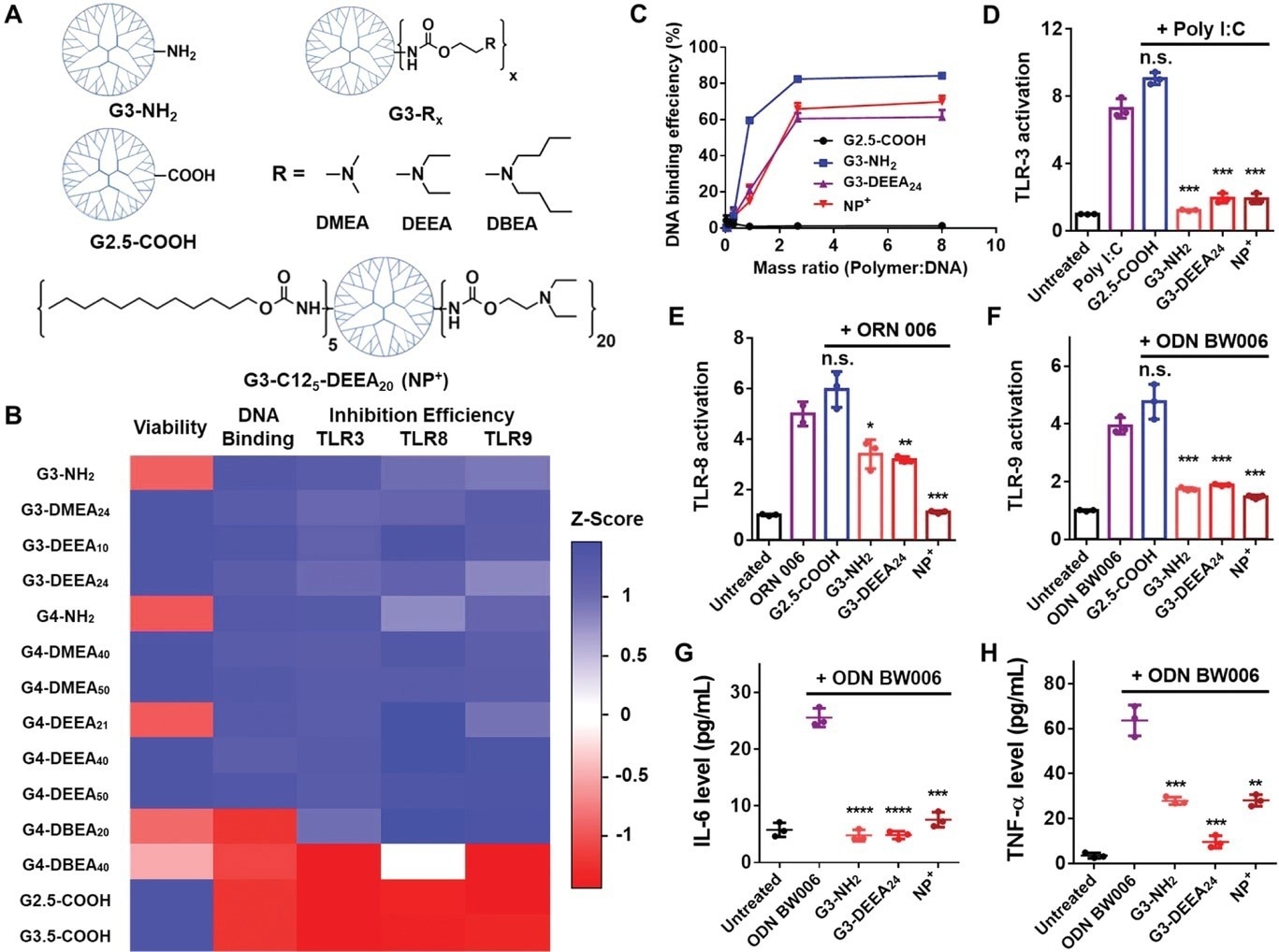
Figure 1. Structures and characterization of the PAMAM dendrimer derivatives. A) Top: Chemical structures of the PAMAM derivatives tested initially. Bottom: Structure of G3-C125-DEEA20, which was selected after additional optimization for the preparation of the nanoparticles. “NP+” is used as an abbreviation for these cationic nanoparticles. B) Heatmap of the Z scores for the different attributes of the PAMAM derivatives. Higher Z scores (blue) indicate better cell viability, DNA binding, or TLR3/8/9 inhibition. C) DNA-binding efficiency of different PAMAM derivatives and G3-C125-DEEA20 nanoparticles at different polymer:DNA mass ratios. D–F) TLR3/8/9 activation assays showing inhibition of nucleic acid agonist-induced TLR activation by the nanomaterials (polymers or nanoparticles). The DNA agonist used to activate HEK-Blue hTLR cells is listed at the top of each panel. The mass ratio of nanomaterials to agonist was 2:1. G,H) IL-6 and TNF-α levels released by RAW 264.7 cells treated with the agonist ODN BW006 with or without the addition of nanomaterials. Comparisons were made with the agonist only group. *p