

| Date | 24th, Oct 2022 |
|---|
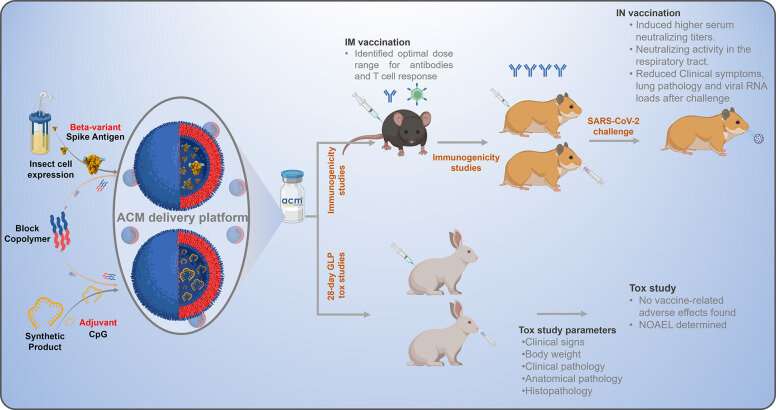 ACM-Covid-19 vaccine preclinical development program. The vaccine consisted of insect cell-produced recombinant spike protein and synthetic CpG adjuvant separately encapsulated in ACM polymersomes for coadministration. Immunogenicity was assessed in mice and hamsters after IM or IN administration. Protection against a live virus challenge was examined in hamsters. Key efficacy readouts are indicated. Safety was evaluated in a repeated dose GLP toxicological study in rabbits. Vaccine-related adverse effects were not detected. Credit: ACS Nano (2022). DOI: 10.1021/acsnano.2c06350
ACM-Covid-19 vaccine preclinical development program. The vaccine consisted of insect cell-produced recombinant spike protein and synthetic CpG adjuvant separately encapsulated in ACM polymersomes for coadministration. Immunogenicity was assessed in mice and hamsters after IM or IN administration. Protection against a live virus challenge was examined in hamsters. Key efficacy readouts are indicated. Safety was evaluated in a repeated dose GLP toxicological study in rabbits. Vaccine-related adverse effects were not detected. Credit: ACS Nano (2022). DOI: 10.1021/acsnano.2c06350
An intranasal vaccine against SARS-CoV-2 could quickly get to the respiratory tract, where the virus most commonly causes symptoms. And a spray or droplets could be a more palatable option for people who fear needles. But so far, only a few countries have approved COVID nasal vaccines. Now researchers report in ACS Nano that they've developed one that can fight off the original virus and two variants in hamsters.
The current batch of injected COVID vaccines have been effective at combating SARS-CoV-2 infection around the globe. But these shots enter the body in the muscle tissue, whereas the virus enters and causes many of the typical COVID symptoms in the respiratory tract. Thus, intranasal immunizations with a spray or droplets could be a better option.
Although India and a couple of other countries have approved intranasal COVID vaccines in recent months, the road to formulating successful intranasal vaccines is not an easy one. For example, AstraZeneca announced this month that its intranasal candidate failed to produce a strong immune response in nasal tissues and offered less systemic protection than the intramuscular version.
So, Madhavan Nallani, Pierre Vandepapeliere and colleagues wanted to formulate an intranasal COVID vaccine that would stimulate an immune response both systemically and in the respiratory tract, and that would also work against SARS-CoV-2 variants.
The researchers based their vaccine on the spike protein from the SARS-CoV-2 beta variant, separately encapsulating the antigen and an immune-stimulating adjuvant into nanoparticles known as artificial cell membrane polymersomes.
They packaged the two components separately so that they could more easily change the spike component to one from another variant if needed. Intramuscular co-administration of the parts produced a strong immune response in both mice and hamsters. When the hamsters injected with the new vaccine were exposed to live virus, however, they still developed an infection.
In contrast, intranasal coadministration in hamsters produced a strong systemic immune response. It also cleared viruses from the respiratory tract and prevented infection-associated lung damage. Regardless of how the vaccine was administered, it provided protection against multiple variants, including omicron. Based on these results, the researchers are now recruiting participants for a Phase 1 clinical trial.
More information: Jian Hang Lam et al, Artificial Cell Membrane Polymersome-Based Intranasal Beta Spike Formulation as a Second Generation Covid-19 Vaccine, ACS Nano (2022). DOI: 10.1021/acsnano.2c06350
Citation: An intranasal COVID vaccine that works against variants in animals (2022, October 24) retrieved 22 November 2022 from https://phys.org/news/2022-10-intranasal-covid-vaccine-variants-animals.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
