Nanotechnology-based drug delivery research has emerged as a field of interest in biomedicine that is expected to elicit therapeutic benefits. An article published in the journal Scientific Reports has presented a nanofiber-based buccal delivery of venlafaxine (VEN) for its controlled release, preventing enzymatic degradation and hepatic metabolism in the gastrointestinal tract (GIT).
Study: Fabrication and characterization of electrospun nanofibers using biocompatible polymers for the sustained release of venlafaxine. Image Credit: Irina Anosova/Shutterstock.com
The prepared nanofibers were composed of poly (ɛ-caprolactone) (PCL) and polylactic acid (PLA) in a 1:1 ratio, the efficiency of which was validated by its drug-loading capacity, morphology, and in vitro drug release.
The ex vivo permeability of the nanofibers was evaluated using chicken pouch mucosa and was subsequently tested for histopathology. Ex vivo permeation highlighted the improved sustained release of the drug and a significant decrease in drug leakage from nanofibers.
Furthermore, scanning electron microscopy (SEM) images revealed the morphology of the prepared nanofibers, and the results showed defect-free uniform nanofibers of PCL, PLA, and PLA/PCL mats. The diameters of the nanofibers were 200–500 nanometers.
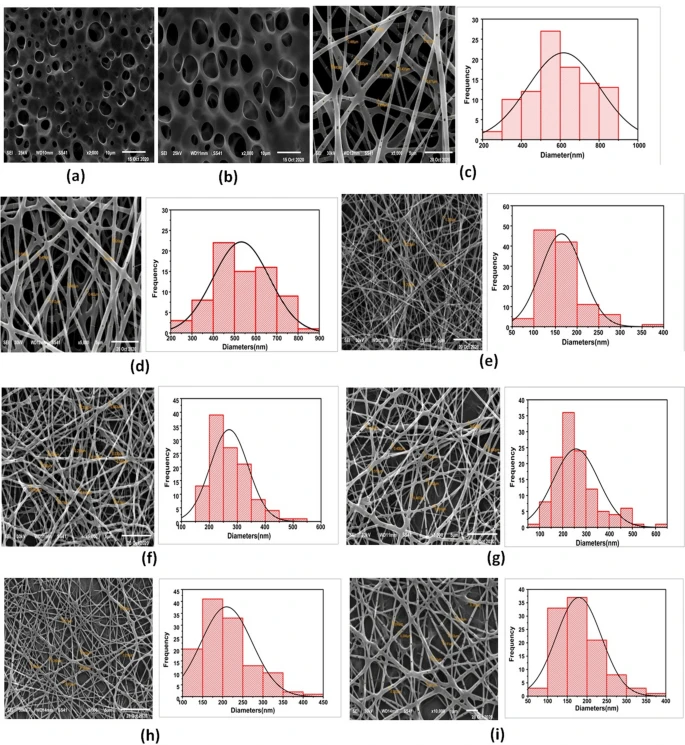
SEM micrographs of (a) PLA cast film, (b) VEN-PLA cast film, (c) 10% PLA NFs, (d) VEN-10% PLA NFs (F1), (e) 10% PCL NFs, (f) 14% PCL NFs, (g) VEN-14% PCL NFs(F2) (h) 10% PLA/PCL composite NFs, and (i) VEN-10% PLA/PCL composite NFs (F3). Image Credit: Hashem, H.M et al., Scientific Reports
Physicochemical characterization of the nanofibers revealed successful loading of the amorphous drug (VEN) into the PLA/PCL nanofibers. The in vitro release of nanofibers showed a 30% burst release of VEN within 0.5 hours, followed by a sustained release of 80% VEN in 96 hours, showing a non-Fickian diffusion mechanism.
Finally, cytotoxicity profiles (IC50) were evaluated in oral epithelial cells for VEN alone, VEN-nanofibers, and nanofibers alone, where the values were 217.55, 250.62, and 440.48 micrograms per milliliters, respectively. Cell toxicity and histopathology studies demonstrated the preservation of the mucosal architecture and preclinical safety of VEN drugs.
Drug Delivery Systems for Controlled Release of VEN
Given the challenges posed by the blood-brain barrier (BBB), treating diseases related to the central nervous system, such as major depression, has become a primary challenge. This barrier prevents the passage of therapeutic molecules with large molecular sizes and low molecular weights, with the exception of small molecules with lipophilic properties.
VEN is a commonly used medication for treating major depression. It is an FDA-approved oral formulation used for pregnant women and adults. VEN is well absorbed from the GIT, with an absorption rate of 92% and an excretion rate of 87% within 48 hours of ingestion. Nanofibers have been utilized as drug delivery systems owing to their facile fabrication and unique properties.
PLA is a renewable material that is widely used in biomedical applications. Its byproducts are biodegradable and extremely safe in the human body and are regarded as suitable hydrophobic media for effective hydrophilic or hydrophobic drug loading.
PCL, which has good drug permeability, a slow degradation rate, and outstanding physicochemical properties for chemical transformations, is also used in medical applications. Consequently, PLA/PCL-blended nanofibers have a synergistic effect owing to their shorter degradation time, superior mechanical properties, and enhanced drug bioactivity.


