

| Date | 20th, Mar 2023 |
|---|
Scientists have discovered that gold nanoparticles supported on a zirconium oxide surface can turn waste materials like biomass and polyester into organosilane compounds, which are valuable chemicals used in various applications. The protocol is a greener and less demanding method for upcycling waste, leveraging the cooperation between gold nanoparticles and the amphoteric nature of the zirconium oxide support.
Supported gold nanoparticle catalyst can upcycle polyester and biomass.
Researchers from Tokyo Metropolitan University have found that gold nanoparticles supported on a zirconium oxide surface help turn waste materials like biomass and polyester into organosilane compounds, valuable chemicals used in a wide range of applications. The new protocol leverages the cooperation between gold nanoparticles and the amphoteric (both acid and base) nature of the zirconium oxide support. The result is a reaction that requires less demanding conditions and a greener method for upcycling waste.
Recycling is a big part of humanity’s solution to the global issue of plastic waste. Much of it is about turning plastic waste into plastic products. However, scientists have also been exploring alternative approaches to encourage the use of waste materials as a resource. This includes upcycling, the conversion of waste material into entirely new compounds and products which can be more valuable than the materials used to make them.
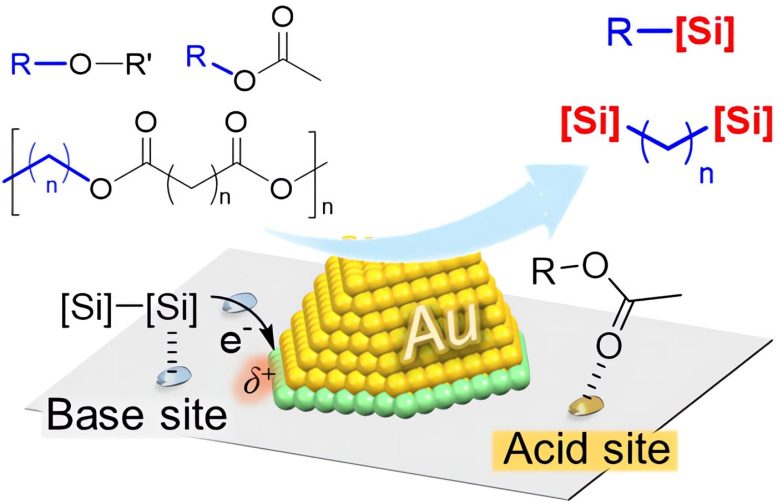
Ethers and esters are reacted with a disilane in the presence of a hybrid catalyst consisting of gold nanoparticles mounted on a zirconium oxide substrate. The presence of the gold nanoparticles and both acidic and basic sites on the support helps convert the ethers and ester groups to silane groups. Credit: Tokyo Metropolitan University
A team of researchers from Tokyo Metropolitan University led by Associate Professor Hiroki Miura has been working on the conversion of plastic and biomass to organosilanes, organic molecules with a silicon atom attached to form a carbon-silicon bond. Organosilanes are valuable materials in high-performance coatings and intermediates in the production of pharmaceuticals and agrochemicals. However, the addition of the silicon atom often involves reagents that are sensitive to air and moisture and require high temperatures, not to mention harshly acidic or basic conditions. This potentially makes the conversion process itself an environmental burden.
Now, the team has applied a hybrid catalyst material consisting of gold nanoparticles supported on a zirconium oxide support. The catalyst takes ether and ester groups, both abundant in plastics like polyester and biomass compounds like cellulose and helps them react with a silicon-containing compound known as a disilane. Under mild heating in solution, they successfully created organosilane groups where the ester or ether group was situated. Through detailed studies of the mechanism, the team found that the cooperation between the gold nanoparticles and the amphoteric (both basic and acidic) nature of the support was responsible for the effective, high-yield conversion of the raw material under mild conditions.
Given that plastic waste disposal often requires combustion or harshly acidic/basic conditions, the process itself already provides an easy route to decompose polyesters under much less demanding conditions. However, the key point here is that the products of the reaction are themselves valuable compounds, ready for new applications. The team hopes that this new route to organosilane production forms part of our pathway to a carbon-neutral future, where plastics do not make their way into the environment, but into more useful products in society.
Reference: “Diverse Alkyl–Silyl Cross-Coupling via Homolysis of Unactivated C(sp3)–O Bonds with the Cooperation of Gold Nanoparticles and Amphoteric Zirconium Oxides” by Hiroki Miura, Masafumi Doi, Yuki Yasui, Yosuke Masaki, Hidenori Nishio and Tetsuya Shishido, 20 February 2023, Journal of the American Chemical Society.DOI: 10.1021/jacs.2c12311
This work was supported by the Program for Element Strategy Initiative for Catalysts and Batteries (ESICB) (Grant Number JPMXP0112101003), the JST FOREST Program (Grant Number JPMJFR203V), Grants-in-Aid for Scientific Research (B) (Grant Number 21H01719), Challenging Research (Exploratory) (Grant Number 22K18927), and Scientific Research on Innovative Areas (Grant 17H06443) commissioned by MEXT, Japan.
